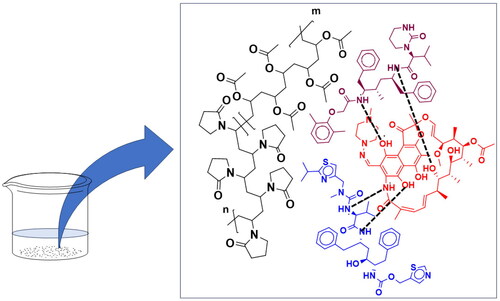
Figures & data
Table 1. Dose of marketed formulations.
Table 2. Chromatographic conditions for the simultaneous estimation of RIF, RTV, and LOP.
Table 3. Solubility parameter calculation for RIF, RTV, LOP and PVP VA using Hansen solubility parameter method.
Table 4. Experimental solubility data of pure RIF, RTV, and LOP (n = 3).
Figure 3. Overlay of DSC thermogram of before and after pH-shifted sample of RIF in presence of RL-SD.
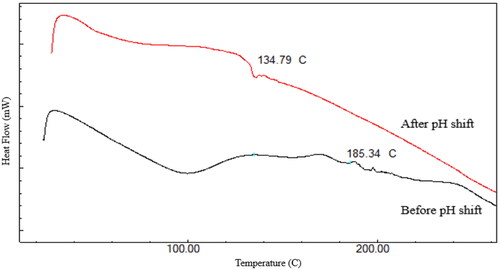
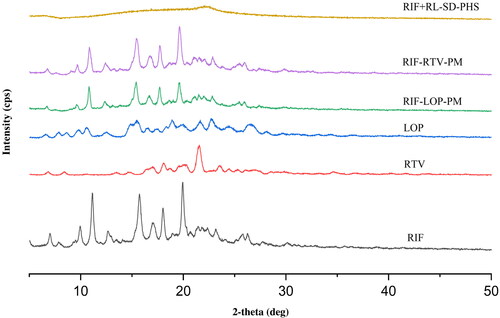
Figure 4. Overlay of FTIR spectra of A. RIF + RL-SD-PHS, B. RIF-LOP-PM, C. RIF-RTV-PM, D. RIF and E. RL–SD.
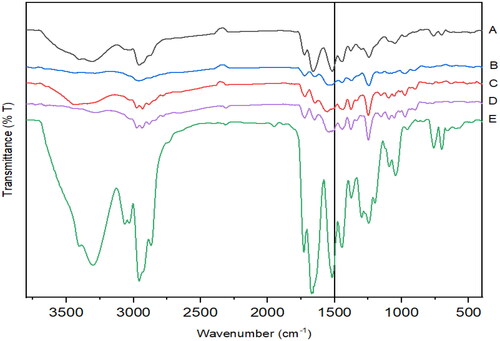
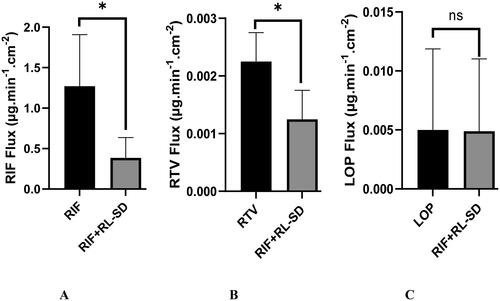
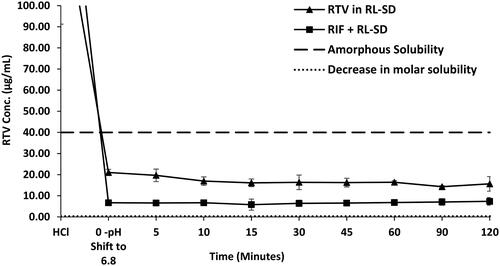
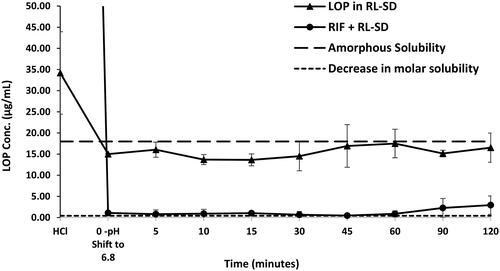
Figure 14. In-vitro flux study of A. RIF in pure RIF & RIF + RL–SD, B. RTV in RL-SD & RIF + RL–SD & C. LOP in RL-SD & RIF + RL–SD (*-statistically significant; n = 3).
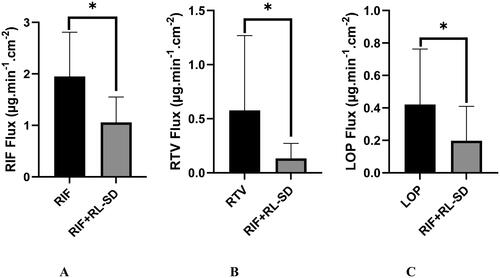
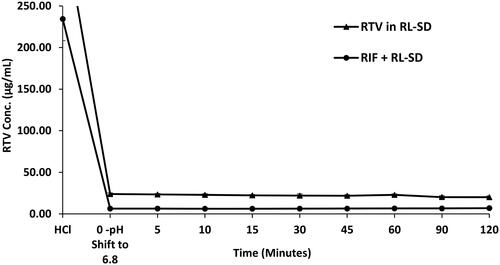
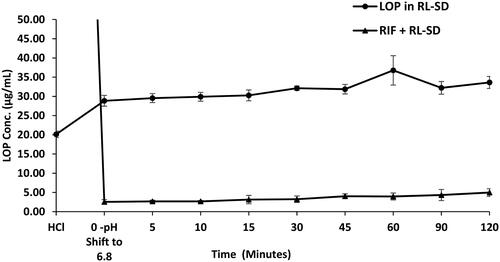
Figure 15. Ex-vivo flux study of A. RIF in pure RIF & RIF + RL–SD, B. RTV in RL-SD & RIF + RL–SD & C. LOP in RL-SD & RIF + RL–SD (*-statistically significant & ‘ns’-statistical insignificance; n = 3).