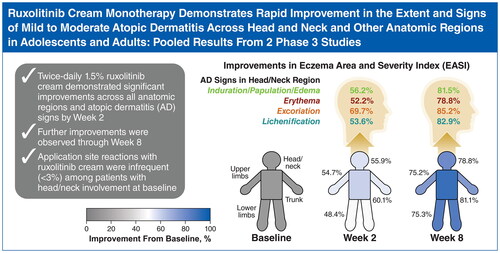
Figures & data
Table 1. Patient demographics and baseline clinical characteristics among patients with head and neck involvement and the overall population.a
Figure 1. LSM percentage improvements from baseline in total EASI anatomic region subscores for head/neck (A), trunk (B), upper limbs (C), and lower limbs (D) in patients applying 0.75% or 1.5% RUX cream versus vehicle. EASI: Eczema Area and Severity Index; LSM: least squares mean; RUX: ruxolitinib. ****p < .0001 vs vehicle.
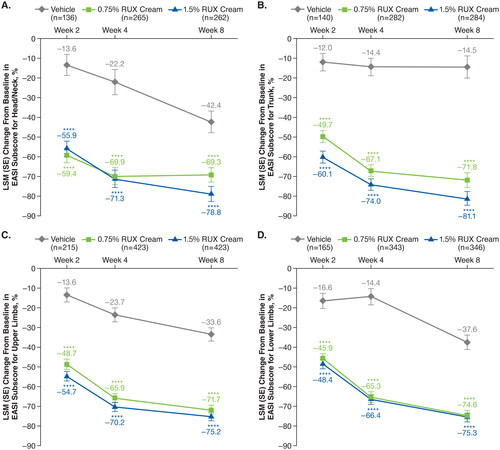
Figure 2. LSM percentage improvements from baseline in EASI anatomic region subscores for induration/papulation/edema (a), erythema (B), excoriations (C), and lichenification (D) in patients applying 0.75% RUX cream versus vehicle. EASI: Eczema Area and Severity Index; LSM: least squares mean; RUX: ruxolitinib. Data are shown for head/neck, trunk, upper limbs, and lower limbs regions. ***p < .001 vs vehicle; ****p < .0001 vs vehicle.
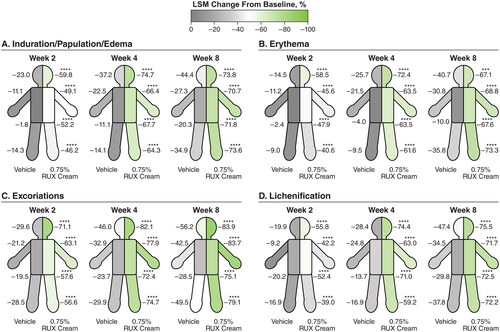
Figure 3. LSM percentage improvements from baseline in EASI anatomic region subscores for induration/papulation/edema (a), erythema (B), excoriations (C), and lichenification (D) in patients applying 1.5% RUX cream versus vehicle. EASI: Eczema Area and Severity Index; LSM: least squares mean; RUX: ruxolitinib. Data are shown for head/neck, trunk, upper limbs, and lower limbs regions. ****p < .0001 vs vehicle.
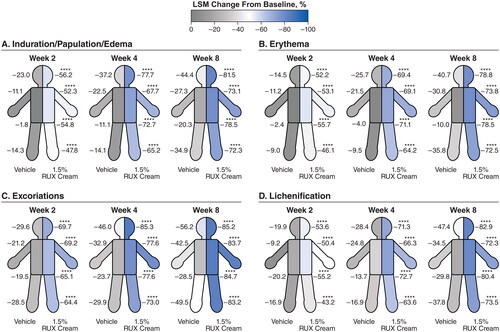
Figure 4. EASI-50 (a), EASI-75 (B), and EASI-90 (C) based on head and neck region subscore in patients with head and neck involvement and composite score in the overall population. EASI-50: ≥50% improvement in Eczema Area and Severity Index score from baseline; EASI-75: ≥75% improvement in Eczema Area and Severity Index score from baseline; EASI-90: ≥90% improvement in Eczema Area and Severity Index score from baseline; RUX: ruxolitinib. ****p < .0001 vs vehicle. †Includes patients with an EASI head and neck region score >0 at baseline; patients with missing postbaseline values were imputed as nonresponders at Weeks 2, 4, and 8. ‡Includes all patients who were evaluable for efficacy; patients with missing postbaseline values were imputed as nonresponders at Weeks 2, 4, and 8.
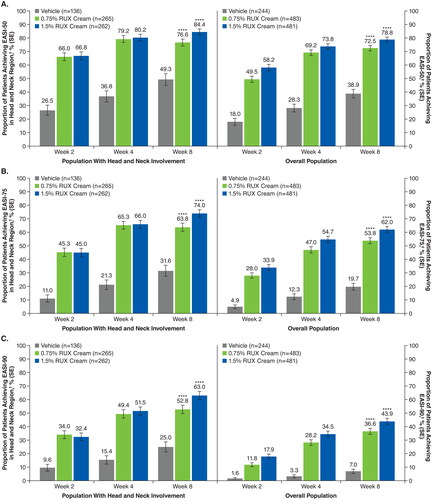
Figure 5. IGA-TS in patients with head and neck involvement and the overall population. IGA-TS: Investigator’s Global Assessment treatment success; RUX: ruxolitinib. ****p < .0001 vs vehicle. †Defined as patients achieving an IGA score of 0 or 1 with an improvement of ≥2 points from baseline. Patients with missing postbaseline values were imputed as nonresponders at Weeks 2, 4, and 8.
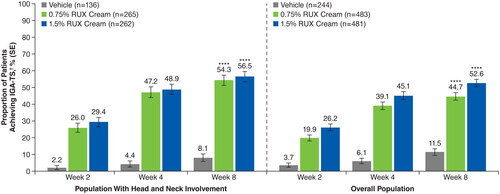
Figure 6. Representative clinical photographs of AD lesions in the head and neck region. AD: atopic dermatitis; RUX: ruxolitinib.
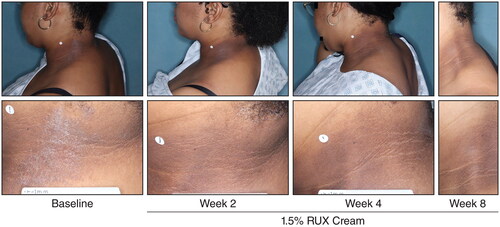
Figure 7. LSM improvements from baseline in DLQI (A) and CDLQI (B) in patients with head and neck involvement and the overall population. CDLQI: children’s Dermatology Life Quality Index; DLQI: Dermatology Life Quality Index; LSM: least squares mean; RUX: ruxolitinib. *p < .05 vs vehicle; **p < .01 vs vehicle; ***p < .001 vs vehicle; ****p < .0001 vs vehicle. †Includes patients with an EASI head and neck region score >0 at baseline.
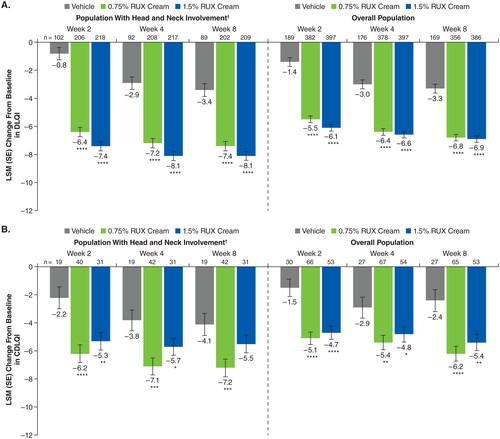
Table 2. Application site reactions in the population with head and neck involvement and overall populations up to Week 8.
Supplemental Material
Download PDF (175.3 KB)Data availability statement
Incyte Corporation (Wilmington, DE, USA) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g., US, EU, JPN). Data will be available for request after the primary publication or two years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.