Figures & data
Table 1. Baseline demographic and disease characteristics of patients with atopic dermatitis (n = 211).
Table 2. Comparison of the background factors between responders and non-responders to upadacitinib 15 mg or 30 mg treatment for atopic dermatitis.
Table 3. Multiple logistic regression analysis for the association of each variable with responders to treatment with upadacitinib 15 mg (n = 159) or 30 mg (n = 52) for atopic dermatitis.
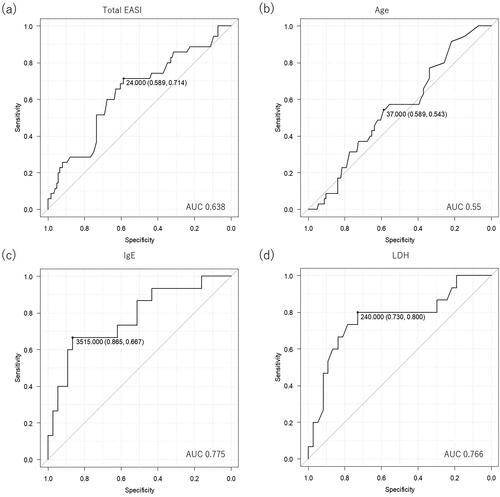
Figure 1. The receiver operating characteristic (ROC) curves for evaluating prediction capabilities of individual parameters for responders to upadacitinib 15 mg or 30 mg treatment. The figures present prediction capabilities of baseline total EASI (a) or age (b) for IGA 0/1 response to 15 mg upadacitinib at week 12, and those of baseline IgE (c) and LDH (d) for the same response to 30 mg upadacitinib.
Supplemental Material
Download PDF (144.7 KB)Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
