Figures & data
Table 1. Baseline demographic and clinical characteristics.
Table 2. Safety outcomes between SR and NSR.
Figure 1. Comparison of PASI response at weeks 4, 12, 24, and 52 between SR and NSR. bIn the NSR group, one patient discontinued the adalimumab treatment and one patient switched to secukinumab. cIn the NSR group, another four patients switched to secukinumab (n = 3) and stekinumab (n = 1), respectively. Among 15 super- responders, 2 (13.3%) patients had a mild degree of psoriasis (PASI = 0.5; PASI =0.6) at week 24 and reached a PASI 100 response again at week 32. dIn the SR group, one patient discontinued the adalimumab treatment, while in the NSR group, eight additional patients, discontinued (n = 4) and switched to IL-17A inhibitors (seckinumab, n = 2; ixekizumab,n = 2), respectively. SR: super responder; NSR: non-super responder; PASI: psoriasis area and severity index. **p-value < .01; ***p-value < .001.
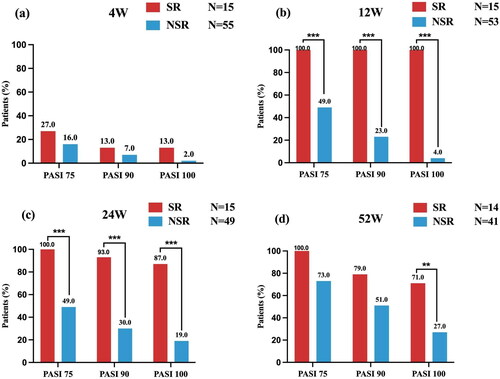
Figure 2. Forest plot of variables associated with the likelihood of reaching PASI 90 and PASI 100 at week 24 and week 52. HDL: high-density lipoprotein; CCI: Charlson Co-morbidity Index; PASI: psoriasis area and severity index; Y/N: yes versus no; M/F: male versus female.
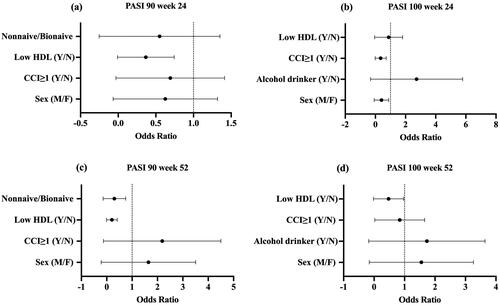
Table 3. Univariate logistic regression analysis: potential predictors for SR.
Figure 3. (a) Adalimumab survival over time between SR and NSR. (b) Adalimumab survival over time between SR-bio-naïve, NSR-bio-naïve and NSR-nonnaïve. SR: super responder; NSR: non-super responder.
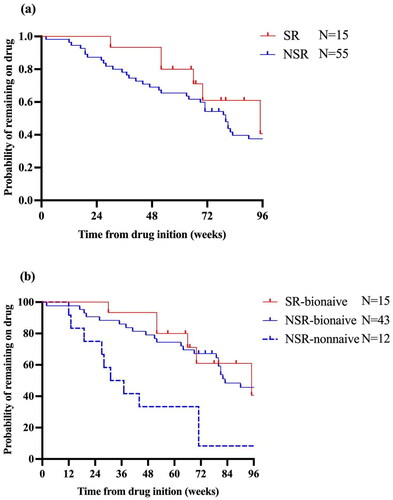
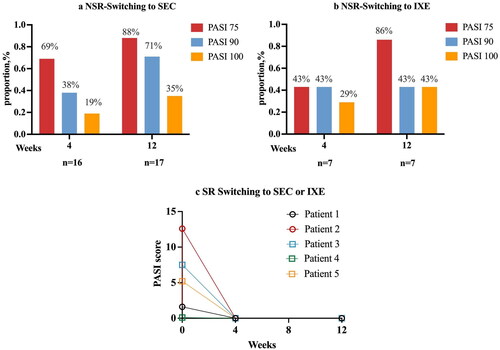
Figure 4. Early effectiveness of NSR and SR after IL-17A inhibitors switching therapy. aPatients who switched to SEC were administrated subcutaneously (150 mg for patients < 60 kg and 300 mg for patients > 60 kg) at weeks 0, 1, 2, 3, and 4, and then every four weeks. bThe dosing regimen for patients who switching to IXE is 160mg at week 0, then 80 mg every 2 weeks for 12 weeks. cIn ©, patients 1 and 2 switched to IXE, patients 3–5 switched to SEC. SR: super responder; NSR: non-super responder; IXE: ixekizumab; SEC: secukinumab.