Figures & data
Table 1. Patient characteristics at baseline.
Figure 1. Mean (a) PASI (0-72) and (b) BSA (0-100) scores at 3, 6, and 12 months.
Error bars depict standard error. The number of patients at each time point are represented within each respective bar.
BSA, body surface area (range 0-100); PASI, Psoriasis Area and Severity Index (range 0-72); PsA, psoriatic arthritis; Pso, psoriasis; SE, standard error.
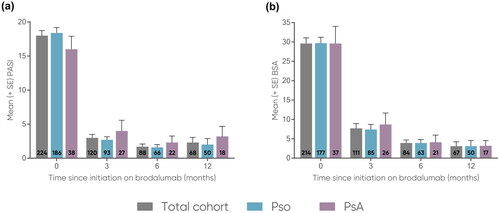
Figure 2. Proportions of patients achieving (a) PASI-75, (b) PASI-90, and (c) PASI-100 at 3, 6, and 12 months.
The number of patients at each time point are represented within each respective bar.
PASI, Psoriasis Area and Severity Index (range 0-72); PASI-75, at least 75% reduction from baseline PASI; PASI-90, at least 90% reduction from baseline PASI; PASI-100, 100% reduction from baseline PASI; PsA, psoriatic arthritis; Pso, psoriasis.
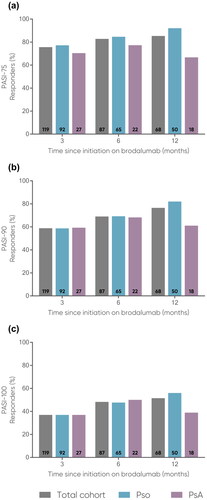
Figure 3. Mean (a) PsA severity (VAS 0-10) and (b) number of swollen (0-74) and number of tender (0-76) joints in PsA patients at 0, 3, 6, and 12 months.
Error bars depict standard error. The number of patients at each time point are represented within each respective bar.
PsA, psoriatic arthritis; SE, standard error; VAS, Visual Analogue Scale.
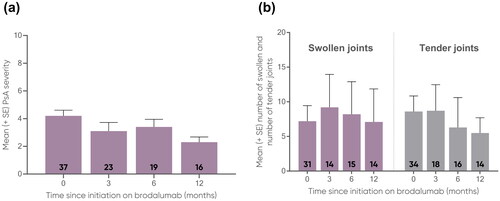
Figure 4. Mean (a) DLQI (0-30) and (b) PBI (0-4) and proportions of patients achieving (c) DLQI 0/1, (d) PBI 1 and PBI
3 at 0, 3, 6, and 12 months.
The number of patients at each time point are represented within each respective bar. For mean DLQI and PBI, error bars depict standard error.
DLQI, Dermatology Life Quality Index; PBI, Patient Benefit Index; PsA, psoriatic arthritis; Pso, psoriasis; SE, standard error.
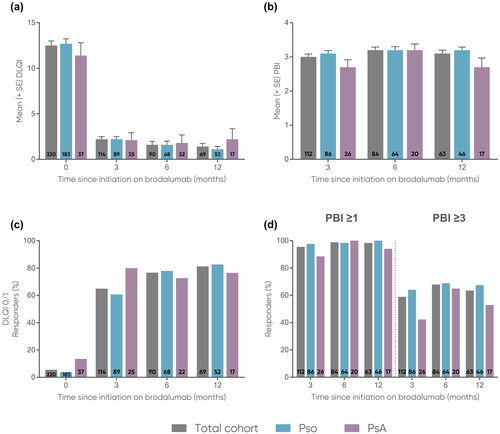
Table 2. Time to discontinuation (for cases with reported stop).
Table 3. Reasons for discontinuation of brodalumab.
Table 4. Adverse events as reason for discontinuation.
Figure 5. Time on brodalumab over 12 months.
Tick marks on connecting lines represent incidences/time points of censoring (i.e. last time of observation, not treatment stop).
PsA, psoriatic arthritis; Pso, psoriasis.
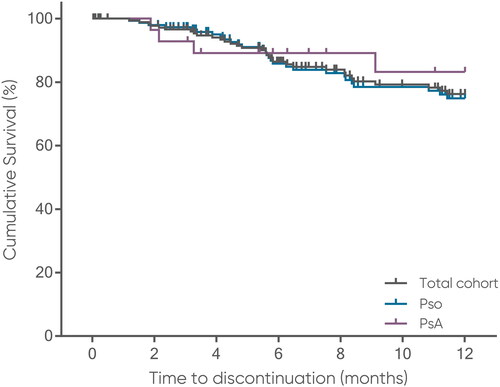
Table 5. Drug survival rate.
Supplemental Material
Download MS Word (52.3 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author, Nesrine Ben-Anaya upon reasonable request.

