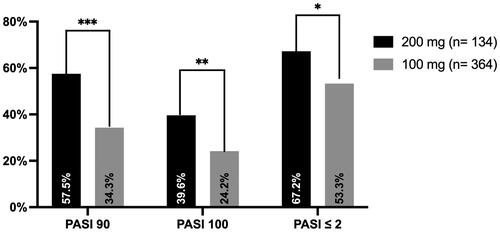
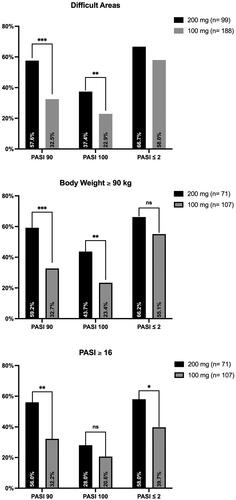
Figures & data
Table 1. Demographic characteristics at baseline of patients treated with tildrakizumab 100 and 200 mg.
Data availability statement
All the patients’ data and information supporting the findings of the study are available from the corresponding author upon reasonable request.