Figures & data
Figure 1. Overview of the skin sensitization AOP. KE1: Covalent binding to skin proteins, the MIE for skin sensitization. Bioavailable, electrophilic chemical must bind covalently to skin proteins, forming a complete antigen recognised by the immune system. KE2: Keratinocytes activation, resulting in upregulation of inflammatory cytokines and chemokines, and induction of cytoprotective gene pathways. KE3: APCs activation and presentation of haptenated proteins to T cells, leading to migration of activated T cells into circulation, completing induction phase of sensitization. KE4: T cell proliferation in response to the haptenated protein presented by APCs, leading to the activation of memory T cells. Adverse outcome (ACD): the clinical manifestation of skin sensitization (elicitation) occurs on subsequent exposure to the same or a cross-reactive chemical. Each KE described above also occurs during elicitation, with some modification. The sensitizing chemical binds covalently to skin proteins, which are then processed and presented to the memory specific T cells. Memory T cells are attracted to the skin site of the exposure by the increased secretion of inflammatory cytokines by keratinocytes. The elicitation phase culminates in the inflammatory response local to the site of exposure to the same (or cross-reactive) chemical the individual has previously been sensitized to. Image: NEXU Science communication.
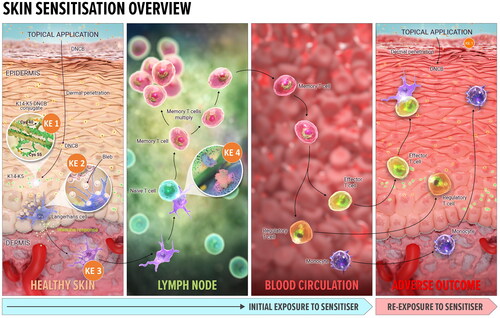
Figure 2. Predictive tools for risk assessment aligned to skin sensitization AOP. Current set of non-animal methods (NAMs) developed for key events in skin sensitization, underpinned by the AOP framework include: the direct peptide reactivity assay (DPRA) (Gerberick et al. Citation2004; Citation2007), amino acid derivative assay (ADRA) (Fujita et al. Citation2014; Yamamoto et al. Citation2015; Fujita et al. Citation2019) as well as kinetic DPRA (kDPRA) (Roberts and Natsch Citation2009; Wareing et al. Citation2017; Natsch et al. Citation2020), all included in the OECD TG 442 C (OECD Citation2023b); KeratinoSens TM (Emter et al. Citation2010; Natsch and Emter Citation2016) and LuSens (Ramirez et al. Citation2014) included in the OECD TG 442D (OECD Citation2022a); human cell line activation test (hCLAT) (Ashikaga et al. Citation2006), U937 cell line activation Test (U-SENS™) (Piroird et al. Citation2015), Interleukin 8 Reporter gene assay (IL 8 Luc assay) (Takahashi et al. Citation2011) and Genomic allergen rapid detection for assessment of skin sensitizers (GARD™skin) (Johansson et al. Citation2011, Citation2013) included in the OECD TG 442E (OECD Citation2023c). In addition to the above tools, numerous predictive chemistry (in silico) tools are also available (dotted box). In vivo evidence (red box) comes from mouse local lymph node assay (LLNA) (OECD Citation2010a) and its variants (OECD Citation2010b, Citation2018) and Buehler and Guinea pig maximization Test (GPMT) (OECD Citation2022b). The LLNA, in fact, addresses KE4, measuring T cell proliferation in treated mice rather than established sensitization.
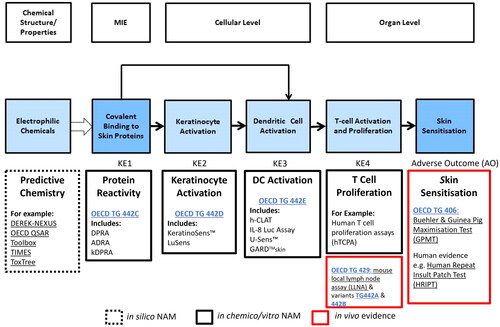
Figure 3. Molecular initiating event (MIE) in skin sensitization by 1,4-dinitro-2-chlorobenzene (DNCB). Besides antigen formation ((a) covalent binding of DNCB to specific cell proteins), additional concomitant and associated events ((b) detoxification, (c) oxidative balance disturbance, (d) lipid peroxidation, (e) covalent modification reversal, (f) activation of the Nrf2 pathway) and (g) covalent protein damage from reactive carbonyl species (RCS, end products of lipid peroxidation) are shown. Image: NEXU Science communication.
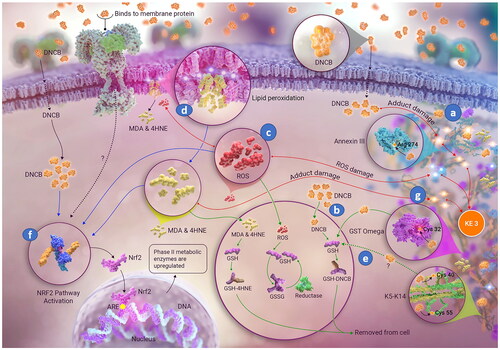
Table 1. Likely phase II metabolic fate of different mechanistic groups of sensitizers.
Figure 4. Structures of some reactive carbonyl species (RCS), end products of lipid peroxidation (LP). The most abundant and most often studied RCS are malondialdehyde (MDA) and 4-hydroxynonenal (4HNE). A large body of literature details extensive investigations of generation, known metabolic routes and effect on certain signaling events, cellular Pool of protein and pathologies associated with production of MDA and 4HNE (e.g. Alzheimer’s disease, Parkinson disease, liver disease, diabetes, cardiovascular disease and cancer) (reviewed by but not limited to) (Ayala et al. (Citation2014)).
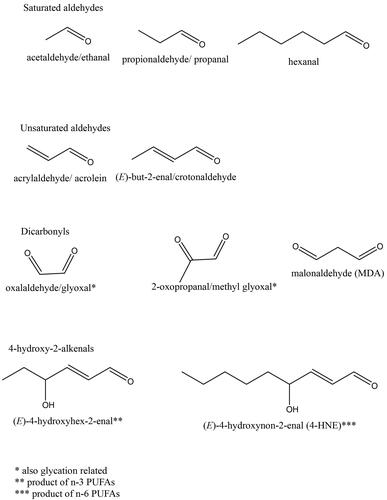
Figure 5. Simplified diagram showing how electrophilic chemicals may interact with cell defense systems, which act in concert to prevent a threshold for sensitization being reached. Electrophilic molecule is likely to encounter cell detoxification mechanisms (k4) faster than be able to covalently modify proteins (k1). It is likely that some type of covalent modifications can be reversed (k3) and subsequently removed by cellular detoxification mechanisms (k4). However, detoxification (k4) may lead to disturbance of redox balance and increase lipid peroxidation (LP, k2), which, in turn, also covalently modifies cellular proteins, potentially speeding up antigen processing and activating unfolded protein response (UPR).
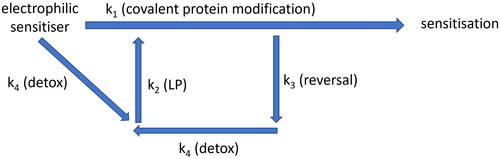
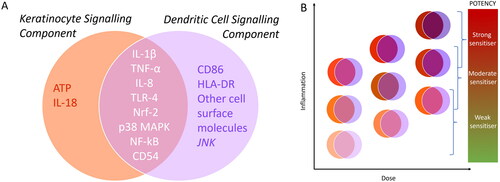
Figure 6. (A) A Snapshot of unique and overlapping signaling components of keratinocytes (KCs) and dendritic cells (DCs) (B) Schematic showing possible dose-dependent interplay and additive effects of inflammatory signals from KCs and DCs feeding into different levels of potency of sensitizing compounds.