Figures & data
Table 1. Formulation development of A. paniculata capsules.
Table 2. Andrographolide contents in A. paniculata capsules (data are expressed as means ± SD, n = 10).
Table 3. Characterization of the phytochemical constituents of A. paniculata powder.
Table 4. Tolerability profile after oral administration of A. paniculata preparations (equivalent to 3 mg/kg of andrographolide).
Figure 2. (a–d). Comparative mean plasma concentration versus time profiles of andrographolide after single and multiple oral administration of A. paniculata alone and in different combination formulations (equivalent to 3 mg/kg of andrographolide) in beagle dogs. Data are presented as means ± SD (n = 4).
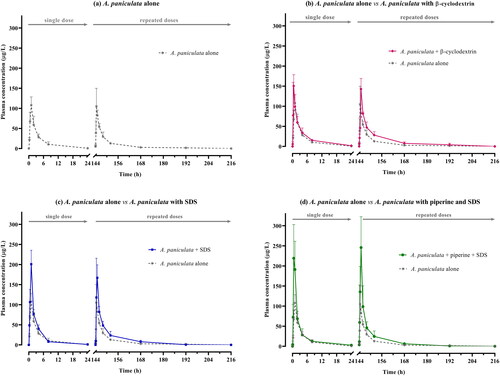
Table 5. Comparative pharmacokinetic parameters of andrographolide after single and multiple oral administration of A. paniculata capsules (equivalent to 3 mg/kg of andrographolide) in beagle dogs.
Figure 3. Cumulative amount of andrographolide in urine after multiple oral administration of A. paniculata formulations (equivalent to 3 mg/kg of andrographolide) in beagle dogs. Data are presented as means ± SD (n = 4).
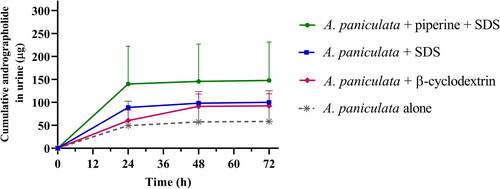
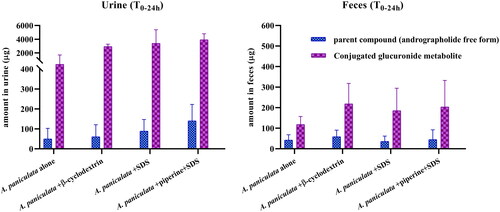
Table 6. Percent recovery of andrographolide elimination in urine and feces after single and multiple oral administration of A. paniculata capsules (equivalent to 3 mg/kg of andrographolide) in beagle dogs (data are expressed as means ± SD, n = 4).
Data availability statement
The datasets generated and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request that needs a consensus from colleagues.