Figures & data
Figure 1. Model structure.
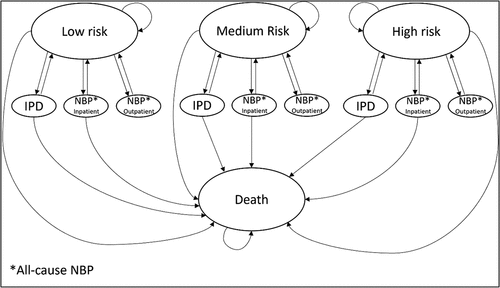
Table 1. Base-case model inputs.
Table 2. Results of base case in 65-year-old adults and adults at high risk aged 60 to 64 years.
Table 3. Breakdown of health outcomes and costs in base case and scenario analysis 1 in 65-year-old adults and adults at high risk aged 60 to 64 years.
Table 4. Scenario analysis (PCV20 vs PPSV23) with different vaccine target population.
Table 5. Scenario analysis (PCV20 vs PPSV23) in 65-year-old adults and adults at high risk aged 60 to 64 years.
Figure 2. Deterministic sensitivity analysis results: tornado diagram.
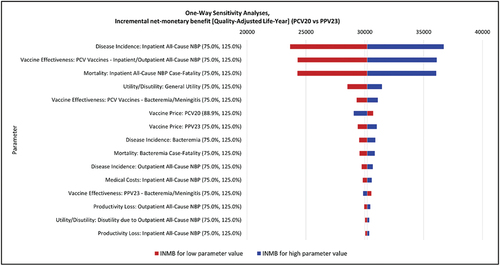
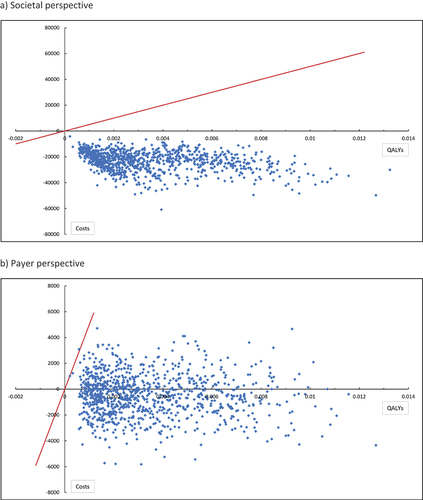
Figure 3. Probabilistic sensitivity analysis results for the cost-effectiveness plane (PCV20 vs PPSV23).