Figures & data
Figure 1. Illustrated potential immune-related Adverse Outcome Pathway for IL-2-mediated hepatotoxicity. Binding of IL-2 to IL-2R expressing cells initiates the AOP (MIE). IL-2 binding induces signaling and proliferation (a.), receptor upregulation (b.), cytokine expression (c.) or interaction with other immune cells (d.; KE1). Consequently, activated immune cells are recruited to the liver sinusoids and adhere to LSECs and/or transmigrate into the perisinusoidal space (KE2). Liver damage occurs by direct or indirect cytotoxic effects or interaction of recruited immune cells with hepatocytes or by impairing the blood flow in the sinusoid lumen (KE3). Finally, impaired liver function or killing of hepatocytes triggers the AO hepatotoxicity (not depicted). Black arrows mark interactions, dashed lines indicate possible interaction points which lack of literature evidence.
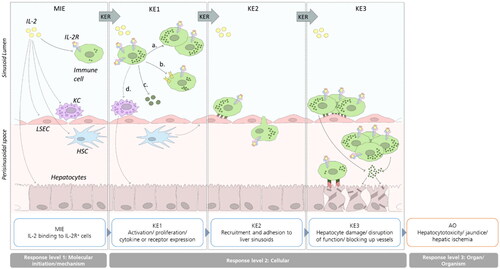
Table 1. Biorelevant molecules and mechanisms related to IL-2 according to the irAOP.
Table 2. Knowledge gaps identified from the irAOP and suggested experimental approaches to fill the gaps.
Figure 2. IL-2 receptor signaling and mechanisms of internalization. (A) The low-affinity receptor consists of IL-2Rα, the intermediate-affinity receptor consists of IL-2Rβ and -γ and the high-affinity receptor of all three subunits. While the low-affinity receptor lacking intracellular signaling domains can act as decoy receptor, the intermediate- and high-affinity receptors signal via MAPK, Pi3K and STAT pathways. (B) Upon IL-2 binding, the ligand-receptor complex is internalized and the IL-2Rβ and –γ subunits are targeted for endosomal degradation, while the –α subunit is recycled to the cell surface. Figure adapted after Spolski et al. (Citation2018).
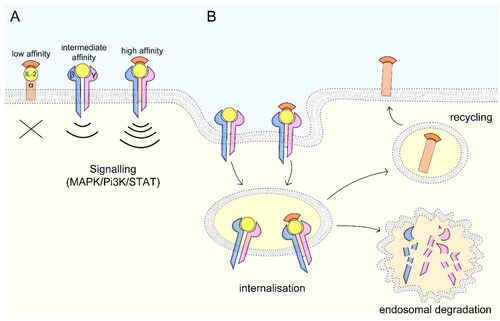
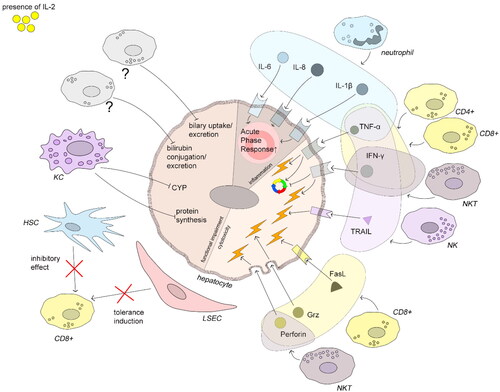
Figure 3. Immune-mediated hepatocyte damage induced by IL-2. All depicted mechanisms follow IL-2 stimulation. Neutrophils produce the cytokines IL-1β, -6, and -8 which increase acute phase protein production. TNFα can act pro-apoptotically but also promotes liver regeneration under certain conditions (see text). TNFα is produced by neutrophils, CD4+ T-, CD8+ T-, NKT, and NK cells. IFNγ inhibits cell cycle progression and induces apoptosis and is produced by CD4+ T-, CD8+ T-, and NK cells. NK cells induce hepatocyte apoptosis by TRAIL-TRAIL-R interaction. CD8+ T-cells induce hepatocyte damage by FasL-Fas interaction and by production of perforin and granzymes. Exogenous IL-2 can both overcome the tolerance induction and impair the inhibitory effect of HSCs. KCs downmodulate protein synthesis and CYP activity. Impaired bilirubin and biliary acid metabolism are an AO of IL-2 therapy in humans, but the key players are not evident from literature. The figure compiles human, murine, and rat data.