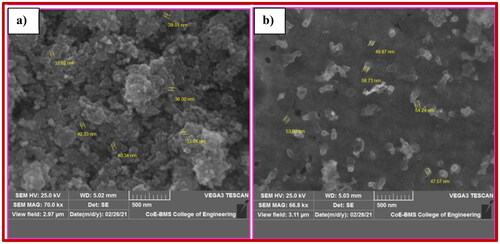
Figures & data
Table 1. List of chemicals, specifications and suppliers used.
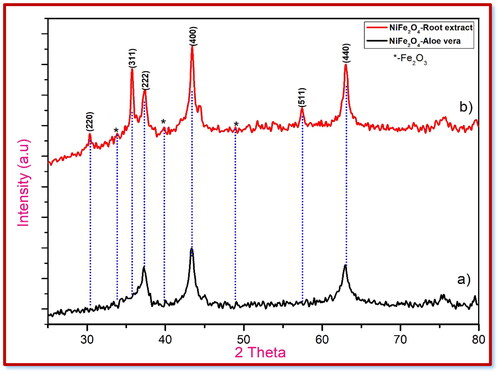
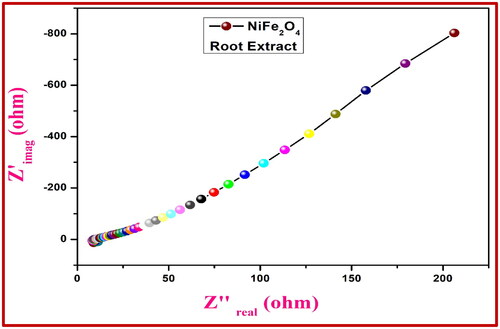
Table 2. Crystalline size and structural constraints of NiFe2O4 nanoparticles.
Figure 7. Proton deficient coefficient and linear fitting of (a) NiFe2O4-aloe vera and (b) NiFe2O4-root extract electrode.
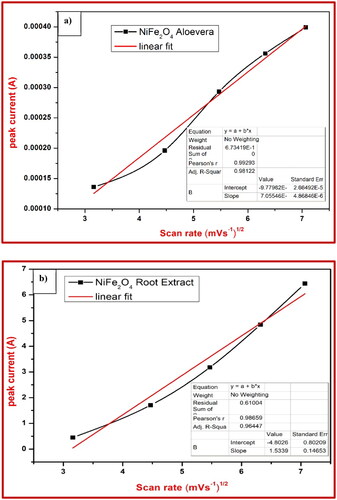
Table 3. Reversibility and proton deficient coefficient of NiFe2O4-aloe vera nanoparticle.
Table 4. Reversibility and proton deficient coefficient of NiFe2O4 root extract nanoparticle.
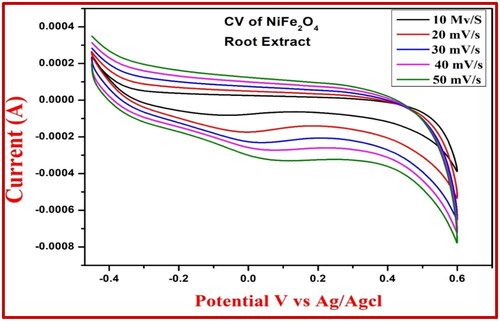
Figure 10. CV response for the NiFe2O4 root extract electrode at 0.1 M HCl of sensor arsenic trioxide.
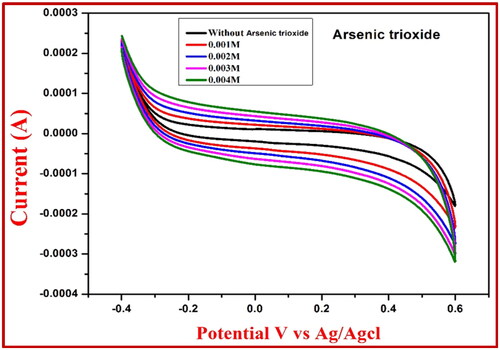
Table 5. Redox reaction values of NiFe2O4 extract using arsenic trioxide sensor.
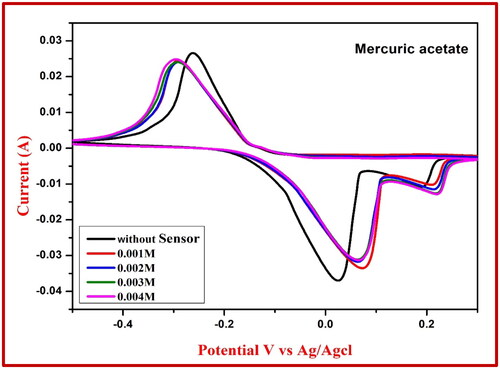
Table 6. Redox reaction values of NiFe2O4 extract using mercuric acetate sensor.
Figure 12. Diffuse reflectance spectra of the synthesised NiFe2O4 from aloe vera and root extract methods.
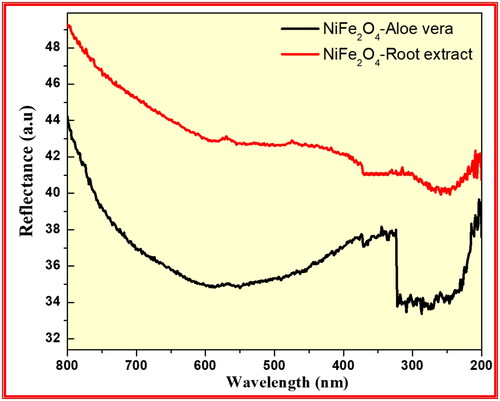
Figure 13. Kubelka-Munk plot transformed reflectance spectra of the synthesised NiFe2O4 from aloe vera and Root extract methods.
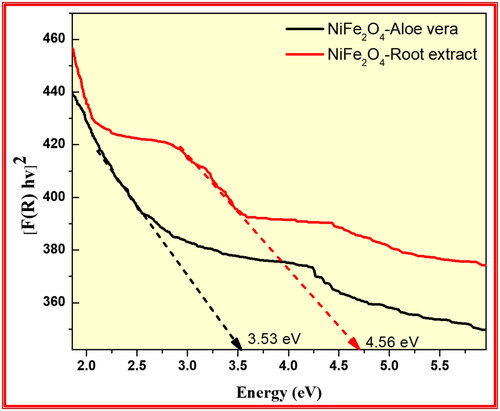
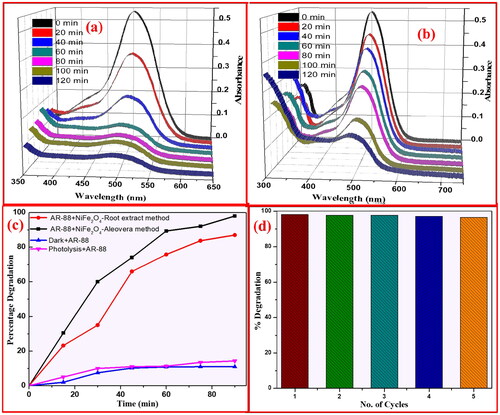
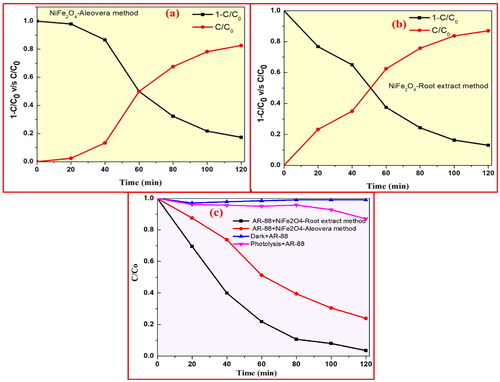
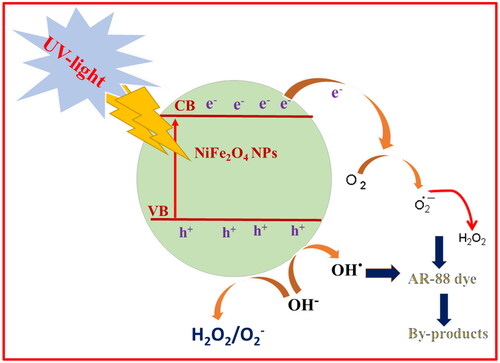
Figure 14. Absorbance of AR-88 (30 ppm) in presence of NiFe2O4 NPs by (a) aloe vera method; and (b) plant root mediated combustion method under UV-light. (c) Plot of % degradation vs time indicating the AR-88 dye photo-degradation capability (d) plot of % degradation vs no. of cycles of AR-88 dye.