Figures & data
Figure 1 Targeting of receptors overexpressed in cancers with oncolytic viruses. Enveloped DNA viruses utilize their surface glycoproteins to bind receptors overexpressed in cancers. Herpes simplex virus glycoprotein D binds herpes virus entry mediator (HVEM) or nectin-1 prior to initiation of host membrane fusion by HSV glycoprotein B. Vaccinia virus and vesicular stomatitis virus bind cell surface glycosaminoglycans (GAGs) and low-density lipoprotein (LDLR) receptor, respectively. Enveloped, RNA viruses Newcastle disease virus and measles virus interact with cell surface sialic acid (SA) and CD46 or nectin-4, respectively, to facilitate entry into host cells. Sialic acid or poly-sialic acid (PolySA) serves as an attachment receptor for non-enveloped DNA viruses such as reovirus and human adenovirus. Junction adhesion molecule-A (JAM-A) acts as the entry receptor for reovirus, whereas coxsackievirus-adenovirus receptor (CAR), CD46, desmoglein-2 (DSG) have been shown to be the entry receptors for adenoviruses. Parvovirus (ssDNA) exploits cell surface transferrin receptor 1 as the entry receptor. Among the non-enveloped RNA viruses Seneca Valley virus, poliovirus, coxsackievirus bind anthrax toxin receptor-1 (ANTXR1), CD155, intercellular adhesion molecule-1 (ICAM-1) or CAR, respectively.
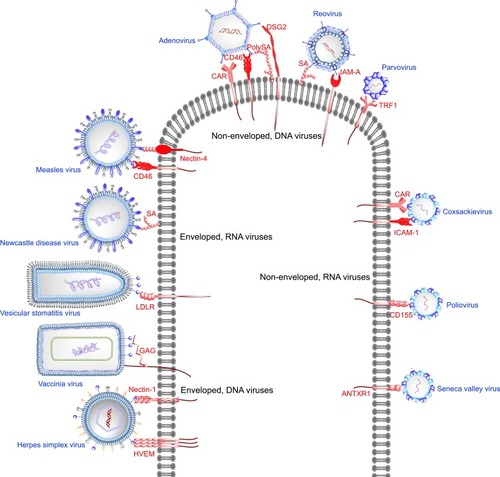
Figure 2 Strategies for retargeting cancers with oncolytic viruses. Oncolytic viral architecture can be modified primarily in three different ways to target cancer-specific receptors. Pseudotyping of lentiviral (LV) envelope glycoproteins with a variant of Sindbis virus envelope protein has enabled successful targeting of P-glycoprotein expressed on melanoma cells. Substitution of vesicular stomatitis virus envelope glycoprotein with a variant glycoprotein from lymphocytic choriomeningitis virus glycoprotein (LCMV-GP) has enhanced the tumor specificity of the recombinant vesicular stomatitis virus (rVSV). Recombinant adenovirus strains have been developed (Ad5lucRGD) by incorporating an RGD moiety required for interaction with integrin receptors overexpressed in cancers. Finally, bispecific soluble adaptors (P-V528LH) have been used in the case of herpes simplex virus (HSV), that includes gD-binding domain of nectin-1 fused to virus and a single-chain antibody with affinity to epidermal growth factor receptor (EGFR).
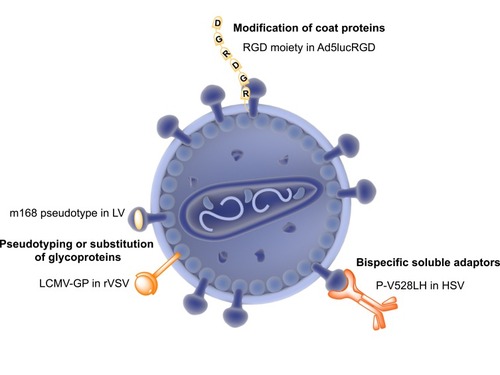
Table 1 Modified Oncolytic Viruses in Clinical Trials
Figure 3 Strategies to avoid virus neutralization. (A) Cell carriers such as monocytes (Reovirus-neutralizing antibody complex), dendritic cells (reovirus), endothelial cells (measles virus), stromal cells (adenovirus) and killer cells (vaccinia virus) remain as most extensively researched solutions to bypass the recognition by neutralizing antibodies. (B) Liposomes have been used to incorporate plasmids of oncolytic viruses such as Telomerase-specific oncolytic adenovirus (pTS). (C) Anti-CD20 and cyclophosphamide (immunomodulators) aid in suppressing the antiviral immune response associated with adenovirus and reovirus treatment. (D) Other solutions to virus neutralization include the use of different serotypes of adenovirus strains, sequestration of pre-existing antibodies using UV–inactivated measles virus (decoy virus), and shielding of vesicular stomatitis virus and adenovirus with coadministration of DNA aptamers and bifunctional protein DE1scFv-pSia, respectively.
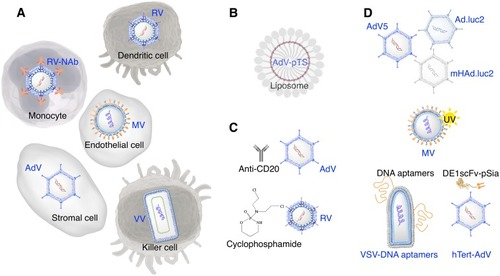
Table 2 Strategies to Avoid Oncolytic Virus Neutralization
