Figures & data
Several sources can be used to obtain dECM, which is extracted and processed via a combination of chemical, physical and/or biological methods, followed by sterilization and quality control steps. For (bio)ink design, the dECM can be combined with cells and used as either a single material or combined with other biomaterials that impart specific properties at rheological, biomechanical and/or biological levels. In the context of skin bioprinting, extrusion- and inkjet-based bioprinting have been used to create acellular and cell-laden constructs as in vitro skin models and grafts for wound healing.
dECM: Decellularized extracellular matrix; ECM: Extracellular matrix.
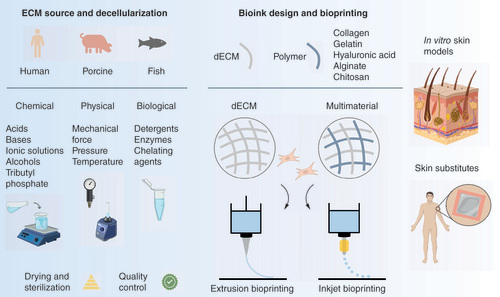
Table 1. Examples of processing methodologies applied for the design of dECM-based bioinks for skin bioprinting.
(A) Quantification of the major ECM components (collagen, GAG, and elastin) after the decellularization process. (B) Representative images of 3D cell-printed in vitro skin equivalents using type I collagen (C-HSE) and skin-derived bioink (S-HSE). Scale bar: 2 mm. (C) Morphological changes occurring in the diverse types of skin grafts during 28 days after the surgery. (D) Bioprinted skin model recreating diabetic hallmarks by the inclusion of perfusable and vascularized hypodermal compartment and augmented diabetic features.
C-HSE: Human skin equivalent using collagen bioink; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GAG: Glycosaminoglycans; IS: Inguinal split-thickness skin grafts; PCL: Polycaprolactone; PDA: 3D-printed dermal analogues; PL: Pelnac Dermal Substitute; ROS: Reactive oxygen species; S-HSE: Human skin equivalent using skin-derived extracellular matrix bioink.
Modified with permission from [Citation10,Citation125,Citation126,Citation145].
![Figure 2. Design and bioprinting of dECM bioinks for skin tissue engineering.(A) Quantification of the major ECM components (collagen, GAG, and elastin) after the decellularization process. (B) Representative images of 3D cell-printed in vitro skin equivalents using type I collagen (C-HSE) and skin-derived bioink (S-HSE). Scale bar: 2 mm. (C) Morphological changes occurring in the diverse types of skin grafts during 28 days after the surgery. (D) Bioprinted skin model recreating diabetic hallmarks by the inclusion of perfusable and vascularized hypodermal compartment and augmented diabetic features.C-HSE: Human skin equivalent using collagen bioink; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GAG: Glycosaminoglycans; IS: Inguinal split-thickness skin grafts; PCL: Polycaprolactone; PDA: 3D-printed dermal analogues; PL: Pelnac Dermal Substitute; ROS: Reactive oxygen species; S-HSE: Human skin equivalent using skin-derived extracellular matrix bioink.Modified with permission from [Citation10,Citation125,Citation126,Citation145].](/cms/asset/a48e71d1-85fb-452e-9649-c08f1a820ec2/idpm_a_12366990_f0002.jpg)
(A) Manipulation of the bioprinted dECM patch (i) and its ability to fit to the wound bed (ii). (B) Effect of gelatin incorporation on the printability of dECM inks and comparison to Pluronic F 127 ink (scale bar: 10 mm). (C) Schematic illustration of the design, bioprinting and implantation of ADSCs-laden ECM–GelMA–HAMA constructs. (D) Macroscopic images of the wound size after treatment with Alg/gel and 5%ECM-Alg/gel 3D printed scaffolds during 21 days upon implantation. (E) Schematic illustration of the process of fabrication of the skin model of hypertrophic scar.
ADSC: Adipose-derived stem cells; Alg: Alginate; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GelMA: Gelatin-methacryloyl; HAMA: Methacrylated hyaluronic acid; PCA: Preformed cellular aggregates.
Modified with permission from [Citation7,Citation14,Citation79,Citation161,Citation162].
![Figure 3. Design and bioprinting of multimaterial dECM-containing bioinks for skin tissue engineering.(A) Manipulation of the bioprinted dECM patch (i) and its ability to fit to the wound bed (ii). (B) Effect of gelatin incorporation on the printability of dECM inks and comparison to Pluronic F 127 ink (scale bar: 10 mm). (C) Schematic illustration of the design, bioprinting and implantation of ADSCs-laden ECM–GelMA–HAMA constructs. (D) Macroscopic images of the wound size after treatment with Alg/gel and 5%ECM-Alg/gel 3D printed scaffolds during 21 days upon implantation. (E) Schematic illustration of the process of fabrication of the skin model of hypertrophic scar.ADSC: Adipose-derived stem cells; Alg: Alginate; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GelMA: Gelatin-methacryloyl; HAMA: Methacrylated hyaluronic acid; PCA: Preformed cellular aggregates.Modified with permission from [Citation7,Citation14,Citation79,Citation161,Citation162].](/cms/asset/96fd6a13-76b8-4326-8947-ea2fc0ec579c/idpm_a_12366990_f0003.jpg)
