Figures & data
Figure 1. LEAP-014 study design.
(A) Part 1. (B) Part 2.
†TP chemotherapy may only be administered to patients in China, Hong Kong, Republic of Korea, and Taiwan. A maximum of 10% of patients enrolled in the study are permitted to receive TP chemotherapy.
CPS: Combined positive score; ESCC: Esophageal squamous cell carcinoma; FP: Cisplatin plus 5-fluorouracil; iv.: Intravenous; mFOLFOX6: Oxaliplatin plus 5-fluorouracil plus leucovorin; OS: Overall survival; PD-L1: Programmed death ligand 1; PFS: Progression-free survival; PO: Orally; Q2W: Every 2 weeks; Q3W: Every 3 weeks; Q6W: Every 6 weeks; QD: Once daily; ROW: Rest of world; TP: Paclitaxel plus cisplatin.
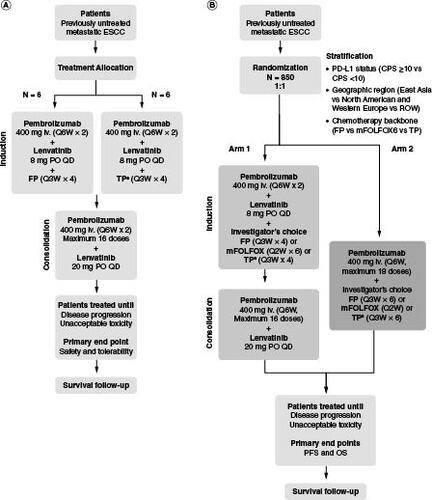
Table 1. Eligibility criteria for the LEAP-014 study.
