Figures & data
Table I. Demographic- and basic clinical data for patients in CRS + HIPEC + EPIC group.
Table II. Demographic- and basic clinical data for patients in systemic chemotherapy treatment group.
Table III. Surgical procedures, tumour burden, completeness of surgical resection, preoperative and perioperative chemotherapy treatment for 10 patients in the CRS + HIPEC + EPIC group.
Table IV. Treatment characteristics of the 10 patients in the systemic chemotherapy treatment group.
Table V. Costs collected in the study.
Figure 1. Overall survival in the CRS + HIPEC + EPIC group and systemic chemotherapy treated group. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; EPIC, early postoperative intraperitoneal chemotherapy; Systemic chemo, systemic chemotherapy treated group.
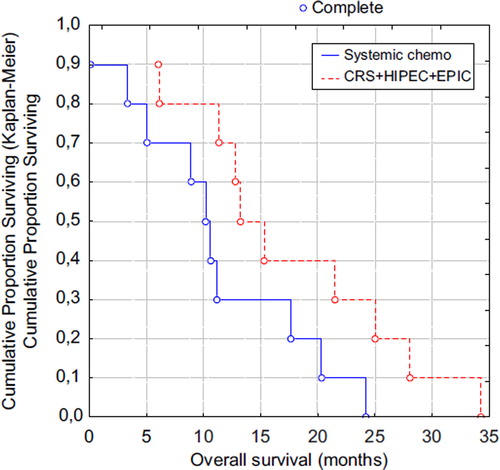
Table VI. Cost per life-years gained and per QALY gained.
Figure 2. Mean costs for the CRS + HIPEC + EPIC group and systemic chemotherapy treated group. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; EPIC, early postoperative intraperitoneal chemotherapy.
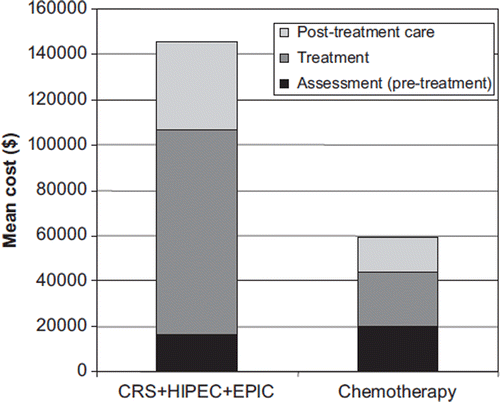
Table VII. Main drivers of the cost during the treatment period.