?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Capsule
We studied the diet of Little Tern chicks in the UK using new data collected as part of the EU LIFE-funded Little Tern Recovery Project (2014–2018) (the LIFE Project), which shows that, despite a wide variety of prey items being recorded, diet at UK colonies is dominated by two types of lipid-rich marine fish: sandeel Ammodytes spp. and clupeid species (Atlantic Herring Clupea harengus and European Sprat Sprattus sprattus).
Aims
To analyse data on the diet of Little Tern chicks from the LIFE Project and assess what additional insight these provide in comparison to the findings in previous literature.
Methods
Timed chick-feeding observations were made at 12 English and Welsh Little Tern colonies between 2014 and 2018 and compared to descriptions of chick diet from other UK studies (published and unpublished).
Results
Chick diet data from the LIFE Project were dominated by lipid-rich marine fish, principally sandeels and clupeid species (82% of all recorded prey items). Adult Little Terns feeding chicks with crustaceans or other invertebrates were recorded at 75% of colonies, but there was no evidence that these routinely made up a substantial proportion of chick diet. We found no significant inter-annual differences in diet composition between individual colonies. However, analysis of records of chick diet over a longer time series (Long Nanny colony in Northumberland, with data available for 17 of the years between 1998 and 2018) showed some significant differences in diet composition between years.
Conclusion
Although Little Terns are generalist feeders, 82% of the prey diet consisted of two prey types: sandeels and clupeids. This may leave existing UK Little Tern colonies vulnerable to any future climate change impacts affecting either the distribution or nutritional quality of their main prey species.
Introduction
The importance of feeding studies in animal ecology is widely recognized (Nielsen et al. Citation2018). Seabirds are particularly well represented in feeding studies due to their position at the top of many marine food chains (Paiva et al. Citation2006a, Paiva et al. Citation2006b, Parsons et al. Citation2008), and because changing patterns of feeding may indicate changes in the wider marine ecosystems, such as declines in the abundance or size of certain fish species (Wanless et al. Citation2005).
The Alternative Prey Hypothesis states that generalist predators will utilize alternative prey as the availability of their main prey declines (Angelstam et al. Citation1984, McKinnon et al. Citation2014, Poysa et al. Citation2016, Reif et al. Citation2001). Diet flexibility in seabirds can reduce the impacts of a shortage of preferred food items if a high abundance of poorer-quality prey are available, meaning that species which can show flexibility in their choice of diet are considered to be less vulnerable to shortages in food supply than species which specialize on a narrow range of prey items (Gaglio et al. Citation2018). However, a diet of low-quality prey may not be adequate for successful breeding (Pierotti and Annett Citation1990), and some access to high-quality prey may still be necessary to ensure chick growth (Paiva et al. Citation2006b). In contrast, the provision of high-quality prey to chicks can bring a range of benefits, including reductions in chick stress and lower parental foraging effort (Čech and Čech Citation2013, Čech and Čech Citation2017).
The Little Tern Sternula albifrons is a UK Amber-listed species (Stanbury et al. Citation2021) and has seen particular conservation effort in recent years. The species has undergone an estimated population decline of 37% in the UK over the last three decades, which has been attributed to reduced productivity and recruitment (Wilson et al. Citation2020). Food availability has been suggested as an important determining factor in the location and size of breeding colonies (Perrow et al. Citation2003). In the UK, Little Terns breed solely on the sea coast, forming colonies on sand or shingle and feeding by plunge diving for prey in shallow waters close to shore. The estimated UK breeding population is 1927 apparently occupied nests (JNCC Citation2021), with 86 separate colonies or sub-colonies (RSPB Citation2019a), although the precise number and size of breeding colonies varies from year to year (Natural England Citation2012). Little Terns are relatively poorly studied compared to other tern species (Cabot and Nisbet Citation2013), owing to the challenges and limitations of high nesting failure, shifting colony locations, precocial and semi-nidifugous chick behaviour, and strong legal protection.
The diet of Little Terns was most recently reviewed by Eglington & Perrow (Citation2014). Data on the diet of this species are available from across its geographic range, including Russia (Snow & Perrins Citation1998), Portugal (Catry et al. Citation2006, Correia et al. Citation2016, Paiva et al. Citation2006a, Ramos et al. Citation2013), Italy (Bogliani et al. Citation1992), Japan (Fujita et al. Citation2009), and Australia (Taylor & Roe Citation2004). Little Terns appear to be opportunistic feeders capable of selecting a wide variety of food items reflecting prey available in the local area (Bogliani et al. Citation1992, Catry et al. Citation2006, Paiva et al. Citation2006a). Studies from across the world have identified prey items as diverse as marine and freshwater fish, crustaceans, annelid worms, marine molluscs and insects, the latter including dragonflies, beetles, and ants (Fasola et al. Citation2002, Eglington & Perrow Citation2014). Within the UK, the Little Tern’s diet is described as consisting mostly of marine fish and invertebrates (Natural England Citation2012, Cabot & Nisbet Citation2013, Eglington & Perrow Citation2014). Since the review of Eglington & Perrow (Citation2014), the EU LIFE-funded Little Tern Recovery Project (2014–2018) (hereafter described as ‘the LIFE Project’) has further investigated food provisioning in Little Tern chicks in the UK. The LIFE project focussed on 26 sites in England and Wales with the overall aim of long-term recovery of Little Terns in the UK (RSPB Citation2019b).
Here, we present data on the diet of Little Tern chicks collected through the LIFE Project between 2014 and 2018, and place this in a long-term context by (i) reviewing relevant literature and (ii) presenting 17 years of diet monitoring data from a colony in Northumberland (northern England). We examine spatial and temporal differences in diet composition and suggest how these data can aid ongoing recovery efforts for Little Terns in the UK.
Methods
The review of chick diet drew on three data sources, which are addressed separately: (i) records of chick-feeding by parent Little Terns, obtained from a literature review of published and unpublished sources, (ii) the LIFE Project’s timed observations of chick-feeding (from 12 English and Welsh colonies), (iii) the LIFE Project’s camera trap observations from a colony at Langstone Harbour in Hampshire (southern England).
Review of existing literature on chick diet
Previous reviews by Fasola et al. (Citation2002) and Eglington & Perrow (Citation2014) were used to identify pre-exiting studies of the Little Tern’s diet at UK locations, with additional information identified through searches of the Web of Science database, Google Scholar, and by direct approaches to the managers of Little Tern sites. Search terms used were ‘Little Tern’, ‘Sternula albifons’ with ‘diet’, ‘food’, ‘foraging’ and ‘provisioning’.
Information for the numbers or relative proportions of different prey were extracted from each of the sources, where available, and were assigned to one of the following categories: (i) sandeel Ammodytes spp., (ii) Clupeidae (Atlantic Herring Clupea harengus and European Sprat Sprattus sprattus), (iii) Goby Gobius spp., (iii) flatfish (Pleuronectiformes), (iv) stickleback Gasterosteidae, (v) other fish species, and (vi) invertebrates. Where prey could not be confidently assigned to a taxonomic category the records were classed as ‘unidentified’.
Collation of the LIFE Project data
Between 2014 and 2018 timed observations of Little Tern chick-feeding took place across 12 English and Welsh colonies as part of the LIFE Project. Data were collected by staff and volunteers at each colony using timed observations of chick-feeding by parent birds. Colony observers carried out feeding surveys independently of each other, leading to variation in the recording effort between locations and years (see for details of colony locations and observation effort). It is estimated that 188 separate broods were subject to feeding observations during the duration of the LIFE Project. Timed observation of chick-feeding is a widely used method for single prey-loading species, such as terns, and allows for the collection of large numbers of feeding records without disturbance to the birds (Barrett et al. Citation2007). A standardized methodology was circulated among monitoring staff at participating colonies (RSPB Citation2015), whereby a nest or brood was selected and observed with telescope or binoculars for a recommended period of at least 60 minutes, while recording prey items brought to the chicks. Individual prey items were usually recorded to the family level, which reduced the need for detailed taxonomic identification. The LIFE Project produced a photo identification guide of common prey species to aid colony staff and volunteers when carrying out the feeding surveys.
Table 1. List of colonies which provide timed chick feeding observation to the LIFE Project and the duration (in minutes) of chick feeding observations made in each year.
Feeding data were supplied by the LIFE Project as spreadsheets or digital scans of reporting forms. The design and format of data capture varied between colonies and between years. These were subsequently collated into a single database and any queries regarding the data provided were resolved directly with the LIFE Project staff.
Additionally, data for chick diet were collected from the additional colony at Langstone Harbour, using camera traps placed at nests during 2015 and 2016. These surveys were separate to the main feeding surveys undertaken by the LIFE Project. The Little Tern colony at Langstone Harbour is located on small islands where the timed feeding observation method would be logistically difficult and lead to disturbance of nesting birds.
The camera traps used in surveys were a mixture of Bushnell and Acorn manufacture. They were deployed on the ground approximately 1 m from nest scrapes and set to record video when triggered by motion. A single camera trap was used on each of the nest scrapes surveyed. Traps were used at four individual nest scrapes during 2015, with video footage captured between 7 June and 19 July. During 2016, camera traps were deployed at six separate nest scrapes, with video footage of chick-feeding captured between the 4 and 9 July. Time- and date-stamped data of individual feeding events were captured by the RSPB site warden for Langstone Harbour and made available through the LIFE Project.
Long Nanny feeding data
In addition to the review of the above information on chick diet, further feeding observations of Little Terns have been made by seasonal wardens at the Long Nanny colony in Northumberland (55°32′24″N, 001°38′16″W) since 1998. The methodology used to record feeding has changed throughout this period. In 1998 and 1999, feeding observations were made on a whole colony basis, while from 2000 onwards one or more individual broods were observed for a fixed period of time. Data on chick diet from 2014 to 2018 were collated as part of the analysis of the LIFE Project data (see above). Data from before this date were extracted from physical copies of annual colony reports held at the National Trust offices at Low Newton, in Northumberland. Items recorded in the chicks’ diet were classified using the categories described above.
Statistical analysis
Data from the LIFE Project were collated for each year and for each of the 12 colonies at which timed observations had been made. Data from the thirteenth colony, Langstone Harbour, did not form part of the analysis as a different field methodology (camera trapping) was used in their collection. Since relative proportions of different prey types found within samples are not independent of each other, compositional analysis was used to examine patterns of chick diet (Aebischer et al. Citation1993). It is important to note that no measure of prey availability was available, therefore the results can be interpreted as dietary differences between or within colonies and years but any patterns could be a result of either prey selection or prey availability or a combination of both.
Prey items recorded in timed observations from the LIFE project were assigned to one of five categories: (i) sandeel, (ii) clupeid, (iii) other fish, (iv) invertebrates and (v) unidentified, the latter comprising unidentified or ambiguously named prey items on recording forms. Unidentified prey were removed from further analysis and the remaining prey categories were expressed as proportions of total observed prey items, with summed values equal to one.
Log ratios were calculated for three of the four prey categories. The fourth prey category (‘other fish’) was used as the denominator for the transformation. The analysis does not depend on which group is used as the denominator group (Aebischer et al. Citation1993). Any zeroes in the dataset were replaced with 0.1 to allow calculation of log ratios.
Log-ratio values for different prey groups were compared using MANOVA to examine the effect of colony location and year, and any interaction between the two. The model was assessed using the Pillai-Bartlett trace statistic (Λ) with values of P < 0.05 considered as significant. Data were tested for multivariate normality using Mardia’s tests. Data were included in the model from all 12 colonies that provided timed observations of chick-feeding to the LIFE project. No significant skew (P = 0.14) or kurtosis (P = 0.51) were found.
Differences between colonies in the proportions of sandeel, clupeids, and invertebrates relative to other prey were analysed further using ANOVA. Model residuals for each prey category were examined using residual vs fitted value plots, residual histograms, Q-Q Plots and Shapiro-Wilks tests. Model residuals conformed with assumption of normality for sandeel (P = 0.39), clupeid (P = 0.09) and invertebrates (P = 0.25).
Observations of Little Terns feeding chicks at Long Nanny pre-date the LIFE Project, and began in 1998. Data from individual feeding surveys were available for 13 of the breeding seasons between 1998 and 2018, while a summary of the overall composition of chick diet (expressed as a percentage of total observed chick diet) was available for an additional four breeding seasons. No quantitative data were available for the years 2000, 2004 and 2006.
Data for chick diet were extracted from annual colony reports and from LIFE Project recording sheets, and observed prey items from each timed count were assigned to the categories: (i) sandeel, (ii) clupeid, (iii) unidentified, (iv) other. This latter category comprised other fish and invertebrates, which were combined due to the low number of records in most years.
Mardia’s tests suggested significant skewness (P < 0.001) in the data from the Long Nanny colony, which could not be addressed by transformation. As such, a Poisson Generalized Linear Model was used to determine if counts of sandeel within observations varied between years using prey data collected from individual timed observations. All statistical analyses were carried out using R (R Core Team Citation2021).
Results
Literature review
We identified 30 sources of information on the diet of Little Terns in the UK for the period up to 2014, of which 28 contained details of chick diet (Table S1). These included 18 additional sources not referenced within the most recent literature review of tern foraging ecology by Eglington & Perrow (Citation2014). All but one of these additional sources came from the grey literature of annual colony reports or student dissertations.
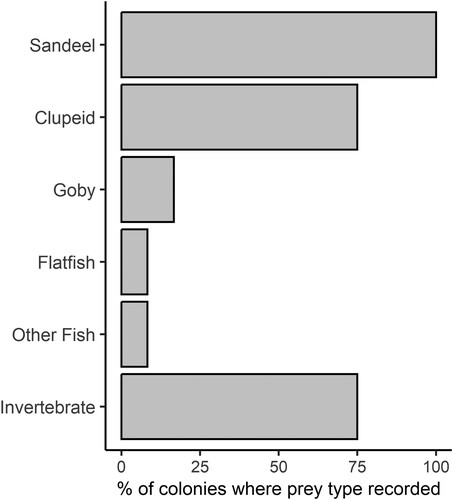
Marine fish dominated dietary records for both adults and chicks (). Within the chick diet, the prey items whose presence was most frequently noted in reports were sandeel (100% of sources) and clupeids (93% of sources). A variety of other fish were noted in the literature, most notably flatfish (Pleuronectiformes), which were mentioned in 50% of reports, and goby, mentioned in 28% of reports. Invertebrate prey items were identified as a component of the chick diet in 51% of available studies, with the majority of items described as ‘shrimp’ (Caridea or Dendrobranchiata) or ‘crustacea’. A median of three different prey types were reported as being present in the chick diet (range = 2–6).
Figure 1. Summary of the frequency of prey types mentioned as forming part of the diet of Little Tern chicks in reports identified by literature review. A total of 28 reports were consulted. Not all reports contained full lists of prey. Reports date from 2014 or earlier. See Table S1 for the sources used.
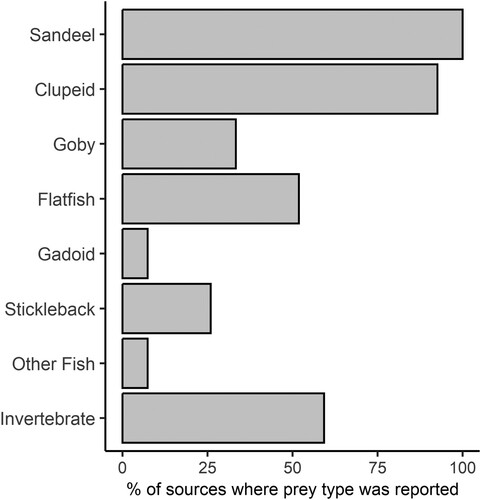
Only 68% of the sources provided information about the relative abundance of individual prey items within the chicks’ diet. Full quantitative data on the diet of chicks could only be confidently derived for three locations: Long Nanny, Easington Lagoons, and the Great Yarmouth and North Denes SPA. Partial quantitative information was given for a fourth site (Gibraltar Point) by Davies (Citation1981) but a complete breakdown of the relative proportions of all observed prey items was not given.
Available numerical data for the chick diet showed a dominance by sandeels and clupeids, although their relative contribution to total diet varied with location. Invertebrate prey made up only a minor component of the chick diet where full quantitative data were provided (typically less than 2%) despite being recorded as a component of the chick diet in over half the available sources. It should be noted that Davies (Citation1981) found that crustacea made up over 90% of chick diet at Gibraltar Point, but we excluded this study from our analysis due to the absence of a full numerical breakdown of the relative proportions of the other prey species mentioned in the paper.
The LIFE Project’s feeding observations
Timed observations made through the LIFE Project produced a total of 2202 chick-feeding records in the period 2014–2018 ((a), ). A median of three different prey types were identified at each colony (excluding unidentified items) across the total duration of the LIFE Project (range = 2–6).
Figure 2. (A): Summary of the numbers of individual prey items recorded through timed feeding observations at the 12 colonies from which observations were made during the LIFE Project (2014–2018). Not all colonies were surveyed in each year. n = total number of prey items recorded at each location. (B): Summary of prey delivered to chicks caught on camera trap footage at Langstone Harbour in 2015 and 2016. n = number of prey items recorded.
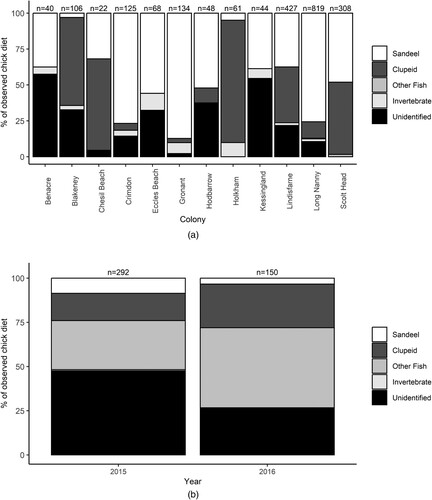
Table 2. Summary of the diet records of Little Tern chicks collected from timed observations by the LIFE Project (2014–2018).
In addition to dietary records obtained through timed observations, an additional 442 records of chick-feeding were captured through camera trap footage at Langstone Harbour during 2015 and 2016 ((b), ). Records from 2015 identified six prey item types, while records from 2016 identified three types of prey (excluding unidentified prey items in both years).
Table 3. Diet of Little Tern chicks at Langstone Harbour, obtained from camera trap images. N is the number of items recorded.
The prey types most frequently recorded during timed feeding observations were sandeels (n = 1 250) and clupeids (n = 562). Sandeels were recorded in the chick diet from all 12 colonies at which feeding data were collected through timed observations ((a)). Other named fish species made up less than 1% of the total prey records. The majority of these other fish species were gobies (n = 6) with a small number of flatfish (n = 2) and a single record of an unsuccessful attempt to feed a Butterfish Pholis gunnellus to a chick.
The dominant prey species identified in camera trap data from Langstone Harbour was gobies (n = 137), with sandeels (n = 30) and clupeids (n = 82) together making up 25% of prey items. Small numbers of flatfish (n = 8) and European Seabass Dicentrarchus labrax (n = 4) were also recorded.
Invertebrates were a minor component of the chick diet in data from the LIFE Project, making up 3% of prey items recorded. Despite the overall low number of records (n = 60), invertebrate prey items were reported in nine of the 12 colonies at which timed feeding observations took place (). The majority of these were identified as ‘shrimp’ on recording forms. In addition to the 12 colonies at which timed feeding observations were undertaken, the Langstone Harbour camera trap data contained only two records of invertebrate food items. Prey items were unidentified in 12% of records from timed observations and 41% of the records from Langstone Harbour camera trap data.
Comparison of the LIFE Project and existing sources
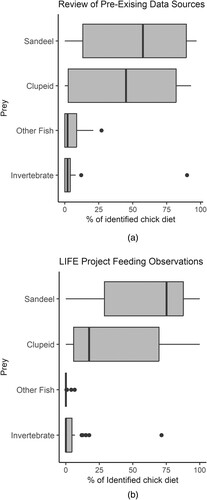
The relative abundance of identified prey items recorded through the LIFE Project was broadly similar to that identified in those existing data sources in which the numbers or percentages of prey were given ((a,b)). The exception to this was prey falling into the category of ‘other fish’ which were recorded almost four times more frequently in the existing studies than in the LIFE Project observations, although it should be noted that 90% of the prey records for this category came from only three of the 14 pre-existing surveys used in the production of these figures.
Figure 4. Boxplots showing the range of contributions which different prey types made to overall chick diet across all UK colonies and years for which numeric data were available from (A): the literature review and (B): the LIFE Project colonies. Calculations of proportions exclude unidentified prey items.
Butterfish (n = 1) and Seabass (n = 4) were both recorded in LIFE Project data (at Long Nanny and in Langstone Harbour camera trap records respectively) and do not appear to have been recorded previously in the diet of Little Terns within the UK. However, both of these species were minor components of chick diet. No records of freshwater/brackish fish, such as sticklebacks, featured in the primary data collected as part of the LIFE Project, but did feature in pre-2014 colony reports from Long Nanny and in reports from Easington Lagoon.
The LIFE Project increased to eight the number of UK Little Tern colonies for which multi-year numeric data on chick diet was available, up from a previous total of three. This included four sites for which no previous information on chick diet has been presented in the available literature.
Variation in diet composition between colonies and years
Chick provisioning data from timed feeding observations were available from multiple years for five locations: Blakeney, Holkham, Gronant, Lindisfarne, and Scolt Head. The composition of the chick diet differed significantly between colonies (Λ = 1.755, P = 0.037) but there was no evidence within individual colonies of a statistically significant difference between years (Λ = 0.499, P = 0.74). ANOVAs showed that, relative to the ‘other fish’ category, there was a significant difference between colonies in the proportion of sandeels observed within the chick diet (F11,14 = 3.32, P = 0.02). No significant inter-colony differences were found in the proportion of clupeids (F11,14 = 1.47, P = 0.25) or invertebrates (F11,14 = 1.34, P = 0.3) relative to prey in the ‘other fish’ category.
The proportion of sandeels observed in the chick diet varied between colonies at which timed feeding observations were made. In nine of the 12 colonies, sandeels made up more than half of the total observed chick diet. The mean percentage of sandeels observed in the chick diet from these 12 colonies across all years was 58.7% (±6.3% se). Only at Blakeney, Chesil Beach, and Holkham was the proportion of sandeels less than 50% in the observed chick diet. Clupeids were the main component of the chick diet at these three sites.
Feeding records from Long Nanny
Data for the observed composition of the chick diet were available for 17 of the 21 Little Tern breeding seasons between 1998 and 2018 (). Between two and six categories of identified prey were observed in each year (median = three). Sandeels were the dominant prey item observed in the chick diet in all years, with the exception of 1998 (). Clupeids were recorded in 15 of the 17 years and were the dominant prey species recorded in 1998, when they accounted for 54% of prey records. The records of gobies, flatfish, and sticklebacks made up a minor component of the observed chick diet (mean = 3%). The exception was in 1999, where together these three fish made up 24% of the total observed prey.
Figure 5. Chick diet composition at Long Nanny from 1998 to 2018 based on annual feeding surveys. n = total number of individual prey items recorded. No quantitative data were available from 2000, 2004, 2006, and 2012.
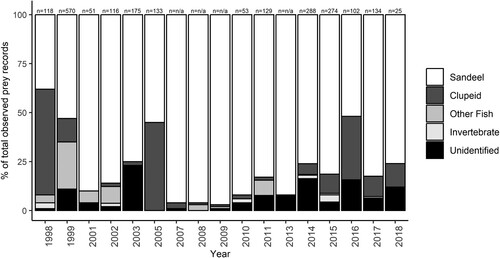
Dab Limanda limanda was identified among flatfish in the 2008 and 2011 feeding surveys, but no detailed taxonomic classification was given in other survey years. Invertebrate prey items (predominantly identified as shrimp) were recorded in five out of the 17 years and made up only a minor proportion of observed prey items (mean of 0.7%).
Data were available on the numbers of individual prey items recorded in 17 years of the 1998 to 2018 period. Comparison of these feeding records showed a significant variation in diet composition between years ( = 66.01, P < 0.001).
Discussion
This study brings together the available data on the chick diet from feeding surveys carried out by the EU-LIFE-funded Little Tern Recovery Project, with data from other UK studies that includes a long-term monitoring project in Northumberland. While a number of pre-existing sources of information on chick diet were available from the UK, these were from a small number of named locations, with limited quantitative multi-year data (Table S1).
The results gathered here confirm the picture of the Little Tern being a dietary generalist that makes use of a wide range of different prey across different colonies and years. However, the overall diversity of prey recorded in the chick diet during any single breeding season was generally low, suggesting a reliance on a small number of prey species. Both sandeels and clupeids are lipid-rich food items and considered to be of high nutritional value for chick development (Green Citation2017, Norman Citation1992, Wanless et al. Citation2005). The frequent occurrence of these two prey perhaps suggests that foraging adults may have been preferentially selecting certain prey types with which to provision chicks. However, in the majority of locations no measure of prey availability was available. The results, therefore, can be interpreted as dietary differences between or within colonies years, but any patterns could result from prey selection or availability, or a combination of both.
The composition of the Little Tern’s diet is generally taken to be a reflection of prey abundance within the foraging distance of colonies, with both Catry et al. (Citation2006) and Perrow et al. (Citation2011) finding that the dominant species observed in the chicks’ diet reflected those found in fish surveys of adjacent waters. Some limited data on prey resources within foraging areas around the LIFE Project colonies were available from 2015 and 2016 fish surveys of Langstone Harbour (MacCallum Citation2015, Citation2016) and from 2016 in the areas adjacent to Long Nanny (Northumberland Inshore Fisheries and Conservation Authority Citation2017). Data were insufficient for statistical analysis of the relationship between the chick diet and available prey, but indicated that the dominant species in the diet coincided with those abundant around the colony. However, the data also indicated that a range of other potential fish prey appeared to be available to foraging birds, which were not regularly recorded in the chick diet. This could suggest that adult terns were not provisioning the full range of potentially available prey to the chicks, and were selectively taking sandeels and clupeids when foraging food for their young. Further study would be needed to confirm this, although some evidence of prey selection by adult Little Terns has previously been found by Phalan (Citation2000) at Kilcoole in Ireland, where gobies were strongly selected for by breeding adults foraging in brackish water, while crustaceans appear to have been largely ignored.
The lack of evidence of inter-annual variation in the composition of the chick diet from the LIFE Project colonies contrasts with the findings of Catry et al. (Citation2006) on the diet of Little Terns in Portugal. This difference may reflect a greater stability in the availability of individual prey species in the foraging areas surrounding UK colonies, although it is also possible that the small sample sizes from a number of colonies has led to lack of statistical power in the LIFE Project data. This explanation is given some support from the evidence of interannual variation in the available chick-feeding data from Long Nanny for the period between 1998 and 2018.
Starvation was recorded as a major cause of chick mortality at Long Nanny in 1999 (Harvey and Shields Citation1999), one of the years with a notably different composition of the chick diet (see ). During 1999, adult Little Terns appear to have compensated for food shortages by bringing a wider variety of fish species to the chicks, as might be expected under the Alternative Prey Hypothesis. The high mortality of chicks in that year suggests that this strategy had limited success. There also appears to have been some starvation-related mortality of chicks at Long Nanny in 2004 (RSPB Citation2004), but unfortunately, no detailed breakdown of chick-feeding is available for that year, so it is not possible to assess how any shortages were reflected in diet composition.
Some uncertainty has been expressed in the literature for Little Terns about the relative importance of fish and crustacea in the diet (Cabot & Nisbet Citation2013). In the UK context, the findings of Davies (Citation1981) are often cited as an example of the importance of crustaceans. However, the current study suggests that, while invertebrates are frequently reported as a component of the chick diet in UK colonies, there is little evidence that they form a major food source. Catry et al. (Citation2006) noted that previous studies in which crustacea were found to be an important component of the chick diet have been at locations where adults foraged over brackish waters. The majority of the UK Little Tern colonies, and all of the colonies studied through the LIFE Project, are in locations where adult foraging is likely to have primarily taken place on the sea coast. This might explain the lack of abundance of crustacea in chick-feeding records and would suggest that Davies’ (Citation1981) results are likely to be unrepresentative of the diet of Little Tern chicks at other UK colonies, at least during those years where alternative prey is abundant.
Similarly, freshwater fish can form an important part of the chick diet in Little Tern colonies elsewhere in the world (Bogliani et al. Citation1992, Citation1994), where these are adjacent to freshwater lagoons or river systems, but the data presented here suggest their importance is limited in the UK. This presumably reflects the foraging habitat available around UK colonies and the presence of higher quality prey items in the form of marine fish.
Summary of findings and potential impacts of climate change for management
The results presented here confirm the generalist nature of the Little Tern’s diet, with a wide variety of prey recorded, but that the chick diet is generally dominated by a small number of food types, principally sandeels and clupeids. The predicted decline of sandeels due to rising sea temperatures is a matter of concern for UK seabird conservation, with changes in the abundance and nutritional quality likely to be affected by climate change (Wright et al. Citation2018, Mitchell et al. Citation2020). The dominance of sandeels within the chick diet at many of the LIFE Project colonies suggests that UK Little Tern colonies are potentially highly vulnerable to any such impacts. The two most likely responses of the Little Tern to any reduction in the quantity or quality of sandeels would be a switch to alternative prey, as predicted by the Alternative Prey Hypothesis, or move of the colony location to areas adjacent to better food resources (Perrow et al. Citation2003). The latter scenario would be particularly unpredictable and could see the abandonment of long-established breeding colonies. Any such changes in breeding location would likely require a flexible and rapid response from site wardens or others involved in the protection of nesting colonies, especially if attempts are made to breed on busy public beaches outside of current conservation management, or on otherwise sub-optimal habitat. Action to pro-actively manage more areas of the coast to provide suitable locations for nesting shorebirds would be advantageous if there is increased movement of Little Tern colonies in the future, in response to changes in food availability, as well as being of benefit to a wider range of breeding coastal birds.
The majority of UK studies of Little Tern diet have used observational techniques to record sightings of feeding. This reflects the high levels of legal protection from disturbance afforded to Little Terns in UK legislation, and the subsequent need for survey methods which are non-invasive and minimize human presence within breeding colonies. However, observation of chick-feeding behaviour is labour intensive and carries a risk that prey items will be misidentified (Barrett et al. Citation2007). There is scope to use a range of other techniques, such as photography (Gaglio et al. Citation2018, Gaglio et al. Citation2017), stable isotope analysis (Ismar et al. Citation2014), DNA barcoding of faecal material (Jo et al. Citation2022), or examination of scales and otoliths in regurgitated food pellets (Correia et al. Citation2016), to bring new insights into the patterns of tern diet. Of these, the use of photography or video to gather additional information is likely to be the technique that could be incorporated into chick diet studies at UK Little Tern colonies, with fewest logistical or resource issues. The camera trap data available from Langstone Harbour indicated that chick diet was dominated by gobies and clupeids, in contrast to the dominance of sandeels and/or clupeids seen at the LIFE Project colonies where timed observations were used. Available information for the abundance of small fish at Langstone Harbour (MacCallum Citation2015, Citation2016) suggests that this difference was likely a true reflection of local prey availability, but additional studies at other sites to compare the results obtained from camera trapping and timed observation techniques would be welcome.
The LIFE Project was notable for producing feeding surveys from a wide geographical spread of English and Welsh Little Tern colonies. However, the recording effort put into gathering data on the chick diet varied strongly between colonies, with relatively low sample sizes being obtained at some locations. Continued and ongoing data collection on the chick diet at colonies would therefore be desirable to help build our current knowledge and to detect any changing patterns of prey availability.
There is limited monitoring of food resources in the foraging areas used by Little Terns in the UK, and, with the exception of some previous studies in Norfolk (Perrow et al. Citation2003, Citation2011), there have been few attempts to link data for food resources and chick provisioning. This would be a valuable research area that would help address some of the uncertainties identified in the current study of the degree to which prey selection influences the composition of the chick diet. Such research could also provide an early warning of future problems, especially if combined with ongoing surveys of chick diet and monitoring of colony productivity.
Supplemental Material
Download MS Word (37.8 KB)Acknowledgements
The EU-funded LIFE + Little Tern Recovery Project (LIFE12 NAT/UK/000869) was coordinated by the Royal Society for the Protection of Birds, in partnership with Cumbria Wildlife Trust, Denbighshire County Council, Durham County Council, Industry Nature Conservation Association, Lincolnshire Wildlife Trust, National Trust, Natural England, Northumberland County Council through the Northumberland Coast AONB Partnership, and Spurn Bird Observatory Trust. We thank the LIFE Project for making their data available and the National Trust for access to the chick provisioning data contained in annual colony reports from Long Nanny.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aebischer, N., Robertson, P. & Kenward, R. 1993. Compositional analysis of habitat use from animal radio-tracking data. Ecology 74: 1313–1325.
- Angelstam, P., Lindström, E. & Widén, P. 1984. Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia 62: 199–208.
- Barrett, R.T., Camphuysen, K., Anker-Nilssen, T., Chardine, J.W., Furness, R.W., Garthe, S., Hüppop, O., Leopold, M.F., Montevecchi, W.A. & Veit, R.R. 2007. Diet studies of seabirds: a review and recommendations. ICES J. Mar. Sci. 64: 1675–1691.
- Bogliani, G., Fasola, M., Canova, L. & Saino, N. 1992. Foraging rhythm and chick diet in Little Terns in three Adriatic coastal wetlands. Avocetta 16: 31–34.
- Bogliani, G., Fasola, M., Canova, L. & Saino, N. 1994. Prey selection by parents and chicks of the Little Tern Sterna albifrons. Avocetta 18: 19–11.
- Cabot, D. & Nisbet, I. 2013. Terns. Harper Collins, London.
- Catry, T., Ramos, J.A., Paiva, V.H., Martins, J., Almeida, A., Palma, J., Andrade, P.J., Peste, F., Trigo, S. & Luis, A. 2006. Intercolony and annual differences in the diet and feeding ecology of Little Tern adults and chicks in Portugal. Condor 108: 366–376.
- Correia, A., Ramos, J.A. & Paiva, V.H. 2016. Identifying the diet of the Little Tern (Sternula albifrons). Waterbirds 39: 318–322.
- Čech, M. & Čech, P. 2013. The role of floods in the lives of fish-eating birds: predator loss or benefit? Hydrobiologia 717: 203–211.
- Čech, M. & Čech, P. 2017. Effect of brood size on food provisioning rate in Common Kingfishers Alcedo atthis. Ardea 105: 5–17.
- Davies, S. 1981. Development and behaviour of Little Tern chicks. Brit. Birds 74: 291–298.
- Eglington, S.M. & Perrow, M.R. 2014. Literature review of tern (Sterna & Sternula spp.) foraging ecology. ECON Ecological Consultancy.
- Fasola, M., Guzman, J.M.S. & Roselaar, C.S. 2002. Sterna albifrons little tern. BWP Update 4: 89–114.
- Fujita, G., Totsu, K., Shibata, E., Matsuoka, Y., Morita, H., Kitamura, W., Kuramoto, N., Masuda, N. & Higuchi, H. 2009. Habitat management of Little Terns in Japan's highly developed landscape. Biol. Conserv. 142: 1891–1898.
- Gaglio, D., Cook, T.R., Connan, M., Ryan, P.G. & Sherley, R.B. 2017. Dietary studies in birds: testing a non-invasive method using digital photography in seabirds. Methods Ecol. Evol. 8: 214–222.
- Gaglio, D., Cook, T., Mcinnes, A., Sherley, R. & Ryan, P. 2018. Foraging plasticity in seabirds: a non-invasive study of the diet of Greater Crested Terns breeding in the Benguela region. PLoS One 13: e0190444.
- Green, E. 2017. A literature review of the Lesser (Raitt's) Sandeel Ammodytes marinus in European waters. Sandy: RSPB.
- Harvey, R. & Shields, L. 1999. Long Nanny Site Wardens Report. Low Newton, Northumberland: National Trust.
- Ismar, S.M.H., Trnski, T.O.M., Beauchamp, T., Bury, S.J., Wilson, D., Kannemeyer, R., Bellingham, M. & Baird, K. 2014. Foraging ecology and choice of feeding habitat in the New Zealand Fairy Tern Sternula nereis davisae. Bird Conserv. Int. 24: 72–87.
- JNCC. 2021. Little Tern Sternula albifrons [Online]. Available: http://jncc.defra.gov.uk/page-2897 [Accessed 20/05/21].
- Jo, H., Jang, J.D., Jeong, K.Y., Gim, J.A., Joo, G.J. & Jeong, K.S. 2022. Prey identification of the Little Tern, Sternula albifrons (Pallas, 1764), by applying DNA barcoding to fecal materials. Sustainability 14.
- Maccallum, L. 2015. Langstone Harbour Small Fish Survey 2015. Hayling Island, Hampshire: Langstone Harbour Board.
- Maccallum, L. 2016. Langstone Harbour Small Fish Survey 2016. Hayling Island, Hampshire: Langstone Harbour Board.
- Mckinnon, L., Berteaux, D. & Bety, J. 2014. Predator-mediated interactions between lemmings and shorebirds: a test of the alternative prey hypothesis. Auk 131: 619–628.
- Mitchell, I., Daunt, F., Frederiksen, M. & Wade, K. 2020. Impacts of climate change on seabirds, relevant to the coastal and marine environment around the UK. MCCIP Science Review 2020.
- Natural England. 2012. Technical Information Note TIN 139 Little Tern: species information for marine Special Protection Area consultations. Peterborough: Natural England.
- Nielsen, J.M., Clare, E.L., Hayden, B., Brett, M.T. & Kratina, P. 2018. Diet tracing in ecology: method comparison and selection. Methods Ecol. Evol. 9: 278–291.
- Norman, D. 1992. The growth rate of Little Tern Sterna albifrons chicks. Ringing Migr. 13: 98–102.
- Northumberland Inshore Fisheries and Conservation Authority. 2017. Long Nanny transitional and coastal waters surveys March 2016 – February 2017. Blyth, Northumberland: Northumberland IFCA.
- Paiva, V.H., Ramos, J.A., Catry, T., Pedro, P., Medeiros, R. & Palma, J. 2006a. Influence of environmental factors and energetic value of food on Little Tern Sterna albifrons chick growth and food delivery. Bird Stud 53: 1–11.
- Paiva, V.H., Ramos, J.A., Machado, D., Penha-Lopes, G., Bouslama, M.F., Dias, N. & Nielsen, S. 2006b. Importance of marine prey to growth of estuarine tern chicks: evidence from an energetic balance model. Ardea 94: 241–255.
- Parsons, M., Mitchell, I., Butler, A., Ratcliffe, N., Frederiksen, M., Foster, S. & Reid, J.B. 2008. Seabirds as indicators of the marine environment. ICES J. Mar. Sci. 65: 1520–1526.
- Perrow, M.R., Gilroy, J.J., Skeate, E.R. & Tomlinson, M.L. 2011. Effects of the construction of Scroby Sands offshore wind farm on the prey base of Little Tern Sternula albifrons at its most important UK colony. Mar. Pollut. Bull. 62: 1661–1670.
- Perrow, M., Tomlinson, M.L., Lines, P., Benham, P., Howe, R. & Skeate, E. 2003. Is food supply behind Little Tern Sterna albifrons colony location? The case of the largest colony in the UK at the North Denes/Winterton SPA in Norfolk. In: R.I., A. (ed.) Proceedings of a Symposium on Little Terns Sterna albifrons. RSPB Research Report 8. Sandy: RSPB.
- Phalan, B. 2000. The diet and early growth of Little Terns (Sterna albifrons) at Kilcoole/Newcastle, Co. Wicklow in 1999. Unpublished BirdWatch Ireland Conservation Report No. 00/1.
- Pierotti, R. & Annett, C.A. 1990. Diet and reproductive output in seabirds – food choices by individual, free-living animals can affect the survival of offspring. Bioscience 40: 568–574.
- Poysa, H., Jalava, K. & Paasivaara, A. 2016. Generalist predator, cyclic voles and cavity nests: testing the alternative prey hypothesis. Oecologia 182: 1083–1093.
- R Core Team. 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Ramos, J.A., Pedro, P., Matos, A. & Paiva, V.H. 2013. Relation between climatic factors, diet and reproductive parameters of Little Terns over a decade. Acta Oecol. 53: 56–62.
- Reif, V., Tornberg, R., Jungell, S. & Korpimaki, E. 2001. Diet variation of common buzzards in Finland supports the alternative prey hypothesis. Ecography 24: 267–274.
- RSPB. 2004. Little Terns in Britain and Ireland, 2004. Sandy: RSPB.
- RSPB. 2015. EU LIFE Little Tern Recovery Project (LIFE12 NAT/UK/000869) Monitoring Sandy: RSPB.
- RSPB. 2019a. Annual Little Tern newsletter 2019. Sandy: RSPB.
- RSPB. 2019b. https://littleternproject.org.uk/ [Online]. [Accessed 16 May 2019].
- Snow, D. & Perrins, C.M. 1998. The Birds of the Western Palearctic. Oxford University Press, Oxford.
- Stanbury, A., Eaton, M., Aebischer, N., Balmer, D., Brown, A., Douse, A., Lindley, P., Mcculloch, N., Noble, D. & Win, I. 2021. The status of our bird populations: the fifth birds of conservation concern in the United Kingdom, channel islands and isle of man and second IUCN red list assessment of extinction risk for Great Britain. Brit. Birds 114: 723–747.
- Taylor, I.R. & Roe, E.L. 2004. Feeding ecology of Little Terns Sterna albifrons sinensis in south-eastern Australia and the effects of pilchard mass mortality on breeding success and population size. Mar. Freshwater Res. 55: 799–808.
- Wanless, S., Harris, M., Redman, P. & Speakman, J. 2005. Low fish quality as a probable cause of a major seabird breeding failure in the North Sea. Mar. Ecol. Progr. Ser. 294: 1–8.
- Wilson, L.J., Rendell-Read, S., Lock, L., Drewitt, A.L. & Bolton, M. 2020. Effectiveness of a five-year project of intensive, regional-scale, coordinated management for Little Terns Sternula albifrons across the major UK colonies. J. Nat. Conserv. 53.
- Wright, P.J., Regnier, T., Gibb, F. & Eerkes-Medrano, D. 2018. Sandeels and their availability as seabird prey. MCCIP Conservation Adaptation report card. Lowestoft: MCCIP.