ABSTRACT
The ability of plants to select effective symbiotic partners is crucial for optimum plant growth. In New Zealand, breeding programmes for white clover (Trifolium repens) have made selections largely based on above-ground characteristics, with little direct attention given to the ability of cultivars to form effective below-ground associations. The ability of three pairs of historical (1930s–1950s), and modern (2000s) cultivars of white clover, to form associations with effective strains of Rhizobium leguminosarum (rhizobia) from a mixture of strains was tested in vitro. First, the efficacy of six individual strains of rhizobia was ranked against all six clover cultivars with shoot biomass used as a direct measure of symbiotic effectiveness for each strain × cultivar combination. Next, each cultivar was inoculated with a mixture of all rhizobia strains at the same cell concentration to examine the identity and frequency of strains found on each host. There was a positive relationship between nodule occupancy and strain effectiveness for historical but not modern cultivars. Cultivars Grasslands Huia and Louisiana (both historical) had nodule occupancy increase with strain effectiveness. This study provides some evidence that historical cultivars may be better able to form associations with effective strains of rhizobia compared with modern cultivars.
Introduction
White clover (Trifolium repens) is an important forage legume in the temperate regions of the world, and is very important for New Zealand agricultural industries (Caradus et al. Citation1995). White clover fixes atmospheric nitrogen via a symbiotic relationship with Rhizobium bacteria (rhizobia) that form nodules on its root system. In the symbiotic relationship between clover and rhizobia, 15%–20% of the photosynthetic carbon obtained by the plant supports the growth of rhizobium-containing nodules, and approximately 15% is exuded into the wider rhizosphere (Kiers and Denison Citation2008). Typically, a plant associates with several rhizobial genotypes, each of which will vary in mutualistic benefit. This can create a situation where some microbes ‘cheat’ by obtaining all the benefits of being in a symbiosis while contributing less to the plant (Denison Citation2021). Therefore, it would make sense for an evolutionary mechanism to be in place to reduce the fitness costs from ‘cheating’ microbes. Kiers and Denison (Citation2008) state that plant sanctions that discriminate among microbial partners based on their symbiotic performance are an important mechanism used to modulate the rhizobia-legume symbiosis.
Theory predicts that microbial cooperation could be maximised if plants preferentially allocated resources to nodules that contain strains of cooperative and high-performing rhizobia (Simms et al. Citation2006; Akçay and Simms Citation2011). In common pea, recent evidence for plant-imposed sanctions on poor performing nodules appeared to be conditional on the quality and presence of available partners (Westhoek et al. Citation2021), while mixtures of Bradyrhizobium strains (typically slow growing) have been shown to reduce the symbiotic benefit received by Acmispon strigosus (Rahman et al. Citation2023). This highlights the importance of competitive strain interaction and host identity when examining the mechanisms stabilising legume-rhizobia mutualisms.
Genetic improvement of pasture plants through plant breeding has been pursued in New Zealand for over 75 years (Easton et al. Citation2002). A recent paper by Weith et al. (Citation2022) highlighted opportunities to breed for improved symbiosis in white clover, however, the breeding focus has historically been aimed at gaining productivity, disease resistance, and forage quality, with little direct attention given to below ground beneficial associations. Therefore, newer germplasm may be in danger of, or may already be, impoverished in their ability to form these associations or to discriminate against poorly performing partners (Liu et al. Citation2020). However, there is little information whether white clover has a tendency for promiscuity or specificity in its rhizobial associations.
Numbers of naturalised Rhizobium strains in soils can reach > 1 × 108 CFU/g with populations varying from 14% to 143% effectiveness (relative to standard commercial rhizobial inoculants in New Zealand) (Wakelin et al. Citation2018; Shi et al. Citation2023) and in competitive ability to nodulate hosts (Irisarri et al. Citation2019). In this background of high rhizobial diversity, the ability of a white clover cultivar to form and maintain associations with effective symbionts would be advantageous. In symbioses with arbuscular mycorrhizal fungi, Trifolium pratense, among other plants showed a degree of specificity in its fungal associations (Ramana et al. Citation2023b), indicating that specificity in other symbiotic relationships may also be present. A study conducted on soybean by Kiers et al. (Citation2007) showed that older cultivars produced more seed than modern cultivars when infected with a mixture of effective and ineffective rhizobia. They also found that when infected by symbionts varying in efficacy, legume defences against poor-quality partners had reduced with continual breeding selection. This raises the question of whether white clover had the ability to discriminate against poor symbiotic partners, and whether that ability has strengthened or weakened over time. No study (to our knowledge) has investigated the relative ability of historical and modern cultivars of white clover to form associations with effective rhizobia.
The objectives of this study were, (i) to rank the efficacy of six strains of R. leguminosarum bv trifolii against six different cultivars of white clover (three historical and three modern); and (ii) to determine if there is variation in effectiveness among the modern and historical cultivars selected. We test the hypothesis that from a mixture of rhizobia strains, nodule occupancy of effective strains will be greater in the historical cultivars compared with modern cultivars.
Materials and methods
Cultivar selection and seed preparation
Six cultivars of white clover were chosen for comparison and comprised three historical cultivars and three modern cultivars representing a range of large, medium, and small leaf size classes (). These were: Grasslands Kopu II (C20133), Grasslands Crusader (C20132), Grasslands Tribute (C21905), Louisiana (C3608), Grasslands Huia (C2027), and Irrigation (C13174). The cultivar seeds were obtained from the Margot Forde Germplasm Centre (MFGC) and the numbers in parentheses are MFGC accession codes. The historical and modern cultivars were matched to each other based on their leaf size class ().
Table 1. Matched pairs of historical and modern cultivars based on leaf size class and growth habits. Date of cultivar release in parentheses.
Seeds were scarified, and surface sterilised by immersion in 70% ethanol for 1 min, then immersed in 4% sodium hypochlorite for 30 s, followed by six rinses in sterile Millipore water for 1 min each. After this, the seeds were left to imbibe and stratify in sterile Millipore water overnight in darkness at 4°C. The following day, seeds were placed onto 10% (w/v) water agar (10 g of Davis Agar per L of water). The plates were wrapped in aluminium foil and placed in an incubator at 22°C for 3–5 days to germinate.
Preparation of rhizobium strains for inoculation
Six strains of rhizobia (Rhizobium leguminosarum bv trifolii: 1302, 316, 451, S12N10, S26N9, TA1) were selected from the Lincoln University Culture Collection based on symbiotic potential data produced as part of the New Zealand Ministry of Business, Innovation and Employment (MBIE) research programme ‘Improved forage legume-rhizobia performance’ (C10X1308). Strain TA1 is a recommended commercial inoculant for white clover, while all other strains had been recovered from nodules of white clover growing in pasture in Canterbury, New Zealand, prior to submission to the Lincoln University Culture Collection.
Pure cultures were streaked onto yeast mannitol agar (YMA; 1 g yeast extract, 4 g mannitol, 0.5 g dipotassium phosphate, 0.2 g magnesium sulphate, 0.1 g sodium chloride, 15 g agar in 1 L reverse osmosis (RO) water, autoclaved for 15 min at 121°C and 15 Psi) and incubated in darkness at 25°C for 4 days to obtain single colonies.
After incubation, a single colony was transferred to a 15 mL tube containing 4 mL of sterile yeast mannitol broth (YMB; 1 g yeast extract, 4 g mannitol, 0.5 g dipotassium phosphate, 0.2 g magnesium sulphate, 0.1 g sodium chloride in 1 L RO water, autoclaved for 15 min at 121°C and 15 Psi) and vortexed for 10 s to mix. A control tube was filled with 4 mL of sterile YMB. Tubes were placed in a shaking incubator for 24 h at 200 rpm at 28°C.
After incubation, the tubes were vortexed for 5 s and 1 mL was then taken from each tube and diluted to a standard inoculum of 106 cells mL−1 in sterile 0.85% w/v NaCl after measuring the optical density at 600 nm (Young et al. Citation2020).
Experiment 1 – symbiotic potential of white clover cultivars with individual strains of rhizobia
White clover seedlings were transferred to 50 mL screw-top Falcon tubes (Neta Scientific, New Jersey, USA) that had been prepared as follows. Each tube was filled with vermiculite pressed down firmly to the 35-mL mark then supplemented with 20 mL of minimal N Mc Knight solution (McKnight Citation1949). The tubes were then sterilised by autoclaving at 121°C for 15 min with lids attached loosely.
The sterilised and germinated white clover seedlings prepared as described above were placed into the tubes using aseptic technique. Seedlings were selected based on uniform vigour and size.
There was a total of eight treatments, one for each strain of rhizobia (six treatments), an added nitrogen control, and a negative control treatment which received no extra nutrition other than the initial addition of minimal N Mc Knight solution that all tubes received. Each replicate, therefore, comprised six cultivars by eight treatments (six rhizobia strains and two controls) and was replicated five times.
Germinated seedlings were inoculated when they were placed in the 50 mL tubes. For those treatments requiring inoculation, 500 μL of the respective diluted strain, prepared as described above, was applied to the hypocotyl and radicle of the plant. The nitrogen control plants received 500 μL of 0.85% NaCl solution after sowing and then 230 μL of sterile 1 M ammonium nitrate solution nine days later. The plants in the negative control treatment received 500 μL of 0.85% NaCl solution when transferred to tubes.
Ten randomly selected tubes were weighed, the average weight taken, and this figure was used as the weight the tubes were watered to throughout the experiment. The plants were placed in a growth room at 16 h light and 8 h dark conditions, at a constant day and night temperature of 22°C for six weeks. Photosynthetically active radiation was provided by four 600 W light emitting diode LED light banks and two 4 × 100W Cob integrated LED light banks. Radiation corresponding to far-red (660–730 nm) and infrared (700 nm to 1000 nm) was provided by six 100 W/240 V R95 incandescent lamps. The plants were watered to weight with sterile Millipore water every three to four days. The experimental design comprised five randomised blocks, each representing a replicate, and each block contained 48 plastic tubes.
Assessment
After six weeks, the shoots and roots were harvested from each plant. Shoots material above the hypocotyl was removed and dried in an oven at 65°C for 72 h and dry weights obtained. The root material below the hypocotyls was washed and the total nodule number was counted, and three random nodules were taken from each plant and surface sterilised. Nodules were surface sterilised by immersion in 70% ethanol for 10 s followed by 2 min in 4% sodium hypochlorite, and four rinses in sterile Millipore water for 30 s each. Once sterilised, each nodule was crushed using a sterile glass rod in a sterile Petri dish. A small drop of sterile Millipore water was placed onto the crushed nodule to create a slurry which was streaked onto yeast mannitol agar (YMA). The plates were incubated at 20°C for 3–5 days and then stored at 4°C until required for direct colony PCR.
The genotype of the strains occupying the nodules was determined by enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) as described by Versalovic et al. (Citation1991), to confirm the presence of the applied strains. The master mix was produced by combining 10 μL of DreamTaqTM DNA polymerase (Thermo Fisher Scientific, Massachusetts, USA), 1 μL of ERIC1 primer (50 μM) sequence (5′ATGTAAGCTCCTGGGGATTCAC3′), 1 μL of ERIC2 primer (50 μM) sequence (5′AAGTAAGTGACTGGGGTGAGCG3′), and 7 μL of sterile water per sample. 19 μL of this master mix was aliquoted into a PCR tube. A sterile plastic 10 μL pipette tip was used to touch a single colony per PCR reaction which was then mixed with the PCR reagents. The PCR thermocycle protocol included an initial denaturation of 3 min at 95°C, followed by 40 cycles of 1 min at 95°C, 1 min at 52°C, and 1 min at 72°C. A final extension of 10 min at 72°C was included to complete the reaction.
The PCR products were resolved by electrophoresis in a 1% agarose gel for 40 min at a constant 120 V. The gel was stained in ethidium bromide for 20 min and then rinsed in water for 10 min. Band patterns were then visualised under UV light in a FireReaderTM (UVITEC) and compared to that generated by a pure culture of the original cultured strains.
Statistical analysis
The impacts of the treatments on shoot dry weight, and nodule number underwent analysis of variance (ANOVA) using GenStat version 16 (VSN International, Hemel Hempstead, UK). The treatment means (excluding nitrogen, and negative controls) for each cultivar were compared using Fisher’s least-significant difference tests (LSD) at a P value of P < 0.1 using the same version of GenStat. Treatment means for each cultivar × strain were used as a direct measure of strain effectiveness for each cultivar in Experiment 2.
Experiment 2 – partner selection by white clover cultivars inoculated with a mixture of rhizobia strains
Plastic tubes containing vermiculite were prepared and white clover seedlings of the six cultivars were sterilised, germinated, and grown for six weeks as described in Experiment 1.
A total of three treatments were applied in this experiment and each cultivar was replicated five times.
Treatment 1: Inoculation with a mixture of the six rhizobia strains described in Experiment 1. This mixed inoculum was prepared by adding 6 mL of each rhizobia strain (strains 1302, 316, 451, S12N10, S26N9, TA1) at a concentration of 1 × 106 cells mL−1 in 0.85% (w/v) NaCl to a 50 mL tube which was supplemented with 14 mL of 0.85% NaCl to reach a final volume of 50 mL. The contents of the tube were mixed using a vortex mixer for 10 s prior to inoculating plants with 500 μL of this rhizobia strain mixture.
Treatment 2: Nitrogen control treatment, which received 500 μL of sterile 0.85% NaCl solution on the day of inoculation, when the rhizobia-treated plants received 500 μL of inoculant. This nitrogen (Positive) control also received 230 μL of sterile 1 M ammonium nitrate solution nine days post inoculation.
Treatment 3: Negative control treatment. These plants also received 500 μL of sterile 0.85% NaCl solution on the day of inoculation of the positive control plants. Plants within the negative control treatment did not receive any further nutrition or additions other than watering to weight (described previously).
White clover seedlings were grown (in the same condition as described in Experiment 1) for six weeks in a trial comprising five randomised blocks. Each block contained 18 tubes, one tube per treatment (inoculated, positive control, and negative control), per cultivar. After the six-week growth period, the shoots were harvested, dried and weighed as described in Experiment 1.
Rhizobial nodule occupancy was determined by taking the first ten nodules from the top of the main tap root. We sampled 10 nodules to standardise our sampling effort across plants. The nodules were isolated, cultured and identified via ERIC-PCR as described in Experiment 1.
Statistical analysis
The effects of the treatments on plant dry weight were subjected to ANOVA using GenStat version 16 (VSN International, Hemel Hempstead, UK).
We counted the number of nodules occupied by each strain for all white clover cultivars and took the sum of counts from all five replicates to establish the total nodule occupancy of all strain × cultivar combinations. Shoot dry weight data from Experiment 1 was used as a direct (continuous) measure of symbiotic effectiveness for each strain × cultivar combination. To test whether historical cultivars had higher nodule occupancy by effective rhizobia strains compared with modern cultivars, we used a generalised linear mixed effect model via the R package ‘LME4’ (Bates et al. Citation2015) as the data fit a Poisson distribution. Nodule count was the response variable and symbiotic effectiveness (Experiment 1 SDW), cultivar age, and their interaction were fixed effects, and cultivar was set as a random effect to account for the variation associated with the different white clover cultivars.
To identify which cultivars of white clover (if any) showed a positive relationship between nodule occupancy and symbiotic effectiveness (defined as SDW produced in Experiment 1) of strains, we fitted a generalised liner model with nodule occupancy as the response, and symbiotic effectiveness (Experiment 1 SDW), cultivar, and their interaction as fixed effects. R version 1.3.1073 (R Core Team Citation2013) was used to create graphs and run statistical models.
Results
Experiment 1 – symbiotic potential of white clover cultivars with individual strains of rhizobia
Out of the six clover cultivars, only Irrigation (historical) and Louisiana (historical) produced significantly different shoot dry weight (SDW) when inoculated with different strains of rhizobia (). Inoculation of Irrigation with rhizobium isolate TA1 produced more SDW than inoculation with any of the other five strains (P = 0.038). For Louisiana, rhizobia strains 451 and 316 produced more SDW (P = 0.013) than the other four strains. There were strong trends for Grasslands Huia (historical) (P = 0.052) and Grasslands Tribute (modern) (P = 0.059) to produce more SDW when inoculated with strain 451 and strain 1302, respectively. By contrast, rhizobium strain did not influence SDW produced by Grasslands Crusader (modern) and Grasslands Kopu II (modern) (P = 0.160 and P = 0.114, respectively).
Table 2. Mean shoot dry weights (mg) of six white clover cultivars inoculated with each of six rhizobia strains.
The total number of nodules was noted for each inoculant treatment, across all live plants. There was a significant cultivar × treatment interaction on total nodule numbers (P < 0.001) as rhizobium strain forming the most nodules varied between cultivars. For example, strain 451 formed the most nodules on Grasslands Huia, but it formed the second least on Grasslands Tribute. There were no nodules found on any positive or negative control plants. Fisher’s least significant difference was used to identify nodule numbers that were significantly different for each cultivar ().
Table 3. Mean number of nodules for six white clover cultivars across each inoculant treatment.
Experiment 2 – partner selection by white clover cultivars inoculated with a mixture of rhizobia strains
When inoculated with a mixture of rhizobia strains, SDW increased for all cultivars regardless of rhizobia identity or nodule occupancy () when compared with single-strain inoculations from Experiment 1. Rhizobium strain 451 was the most common nodule occupant across all the six cultivars of white clover, occupying approximately 55% of the total nodules examined. Strain TA1 was the next most abundant occupying 20% of all the nodules examined ().
Table 4. Mean shoot dry weights of white clover cultivars when inoculated with rhizobia strain mixtures in Experiment 2. Within column values with the same letters are not significantly different at P < 0.05.
Table 5. The number of nodules occupied by each strain of rhizobia for each of the six cultivars of white clover.
To investigate the relationship between cultivar age (historical or modern), influence of rhizobium strain on SDW and nodule occupancy, a generalised linear mixed model was run as described previously. The first comparison was visualised by assigning the cultivars to either historical (n = 3 cultivars) or modern (n = 3 cultivars), then plotting each cultivar × strain combination SDW (Experiment 1, n = 6 strains per cultivar) against the number of nodules harbouring that strain (). It was this relationship against which the generalised linear mixed model was performed. There was a significant interaction between cultivar age and strain-derived SDW, associated with nodule occupancy (P = 0.034). The number of nodules occupied by rhizobia strains that produced higher SDW was higher for historical cultivars compared with modern cultivars, whereas modern cultivars had more nodules occupied with strains producing lower SDW compared with historical cultivars (). When assessing the same data but breaking it down to assess the influence of individual cultivars (n = 6 cultivars × 6 strains), nodule occupancy with better performing cultivar-strain combinations increased only for historical cultivars Grasslands Huia (P = 0.026) and Louisiana (P = 0.005) (). Grasslands Kopu II (modern cultivar), however, showed a strong positive trend, this was not statistically significant (P = 0.093) ().
Figure 1. The relationship between nodule occupancy and cultivar × strain SDW (Experiment 1) for historic (n = 3) and modern (n = 3) cultivars of white clover. Data points for historical cultivars are black and modern cultivars are purple. Trend lines are coloured accordingly and are fitted from the generalized linear mixed effect model.
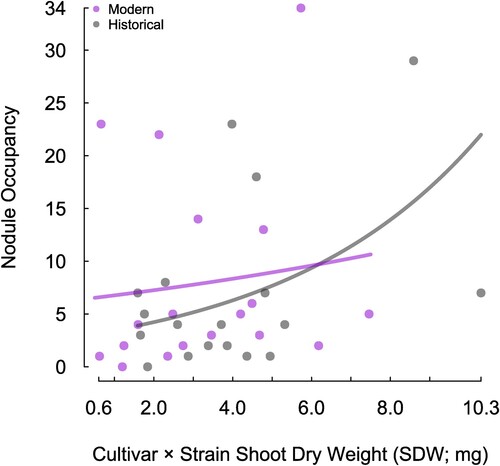
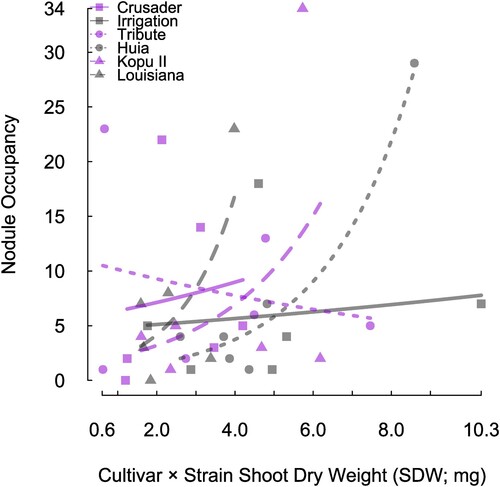
Figure 2. The relationship between nodule occupancy and cultivar × strain SDW (Experiment 1) for all six cultivars of white clover. Modern cultivars are in purple and historical cultivars are in black. Each pair of modern and historical cultivars is represented by the same shape (Crusader and Irrigation = square, Tribute and Huia = circle, Kopu II and Louisiana = triangle). Trend lines are coloured according to cultivar age (historical back, modern purple) and are fitted from the generalised linear model. Each pair of modern and historical cultivar have the same trend line style (Crusader and Irrigation = solid line, Tribute and Huia = dotted line, Kopu II and Louisiana = dashed line).
Discussion
In New Zealand, white clover breeding programmes have focused on above-ground characteristics in selection of new cultivars. This has often been carried out with a background of high nitrogen soil environments, due to applied fertiliser. There is evidence that suggests that breeding has decreased the ability of some legumes, such as soybeans, to defend against poor-quality mutualists (Kiers et al. Citation2007). This is the first study (to our knowledge) to investigate whether historical cultivars of white clover are able to form more associations with effective strains of rhizobia compared with modern cultivars.
In Experiment 1 we found certain strain × cultivar combinations were more effective than others. A recent study conducted by Shi et al. (Citation2023) using 232 isolates of Rhizobium leguminosarum bv trifolii found a similar result where all rhizobia isolates formed nodules, yet the efficacy of rhizobia strains varied significantly among different cultivars of white clover (including Grasslands Tribute). Bacterium-legume specificity has also been demonstrated with Rhizobium meliloti and Medicago sativa genotypes and this was a significant component underpinning variability in plant yield (Chanway et al. Citation1991). This could indicate that the symbiosis between rhizobia and their hosts is one in which genetic differences that can impact the mutualism may occur within both the host and the bacterium (Parker Citation1995; Burdon et al. Citation1999). Weith et al. (Citation2022) showed significant white clover family additive genetic variance for SDW and symbiotic potential using 120 half-sibling families inoculated with commercial strain TA1. This highlighted the host plant genetic potential to influence the symbiosis and how this may be harnessed for improved plant-rhizobium symbiosis through breeding. In our work, the data show that the genotypic variation among the six cultivars and among the selected strains could be a major driver in the variation of symbiotic effectiveness seen in the different clover × rhizobia combinations.
The number of nodules formed by each strain when used as the sole inoculant on each of the six clover species varied. The strain that provided the most benefit did not always produce the most nodules singly. For example, strain S12N10 formed the most nodules on Grasslands Crusader but was the least effective strain in terms of dry weight. A similar result was reported by Sachs et al. (Citation2010) who showed that when effective and ineffective bradyrhizobia were applied to Lotus strigosis, the ineffective strain induced the most nodules per plant. The high number of nodules created by S12N10 and the minimal benefit to the plant host is indicative of a ‘cheating’ strain. A reason for the proliferation of S12N10 nodules on Grasslands Crusader could be that because the host was growing in a low nitrogen environment survival relied on forming any symbiosis, driving the initiation of nodules. Once the nodule had been formed the low N fixation by this strain may have induced the plant to initiate more nodules rather than allocate carbon to the ineffective symbiont. This is a hallmark of the sanction hypothesis (Kiers and Denison Citation2008). In contrast, rhizobium strain 451 formed the most nodules on Grasslands Huia and was also the most effective strain for Huia. This finding supports the sanctions hypothesis (Kiers and Denison Citation2008) which proposes that one of the ways mutualistic interactions are stabilised is when individuals preferentially reward more mutualistic (beneficial) behaviour (West et al. Citation2002).
When the six strains of rhizobia were inoculated as a mixture to the six cultivars, strain 451 outcompeted all other strains being the most dominant nodule occupant across all the six white clover cultivars. Out of the 270 nodules genotyped, strain 451 occupied 149 nodules, and commercial standard TA1 was the next most prominent, occupying 54 nodules. A reason for strain 451’s competitive edge could be its ability to initiate infection and nodulate quickly, and efficiently, providing it an advantage over strains that are slower. Initial nodules are known to apically suppress further nodulation in legumes (Kosslak et al. Citation1983). In the study by Kosslak et al. (Citation1983) examining the effects of pre-exposure of soybean roots to Rhizobium japonicum strains found that the delayed inoculation of a second strain influenced competition. When less competitive strains were inoculated before competitive strains, the proportion of nodules occupied by the less competitive strains was increased. There may be a benefit for the strain which can nodulate first, and perhaps the success of strain 451 to nodulate can be attributed to its ability to out-compete its competitors for initial nodulation.
The dominance of nodule occupation by strain 451 among the cultivars suggested that it is highly competitive relative to other strains when inoculated in a mixture. However, this competitiveness did not always translate into improved symbiotic performance as cultivars such as Grasslands Tribute and Irrigation had highly effective interactions with less competitive strains when inoculated singly. ‘Cheater’ strains, as defined by Kiers and Denison (Citation2008) are strains that are highly competitive for nodulation but provide little or no benefit to plants. However, Experiment 1 also revealed that 451 was an effective strain for some cultivars such as Grasslands Huia. Therefore, it may be possible for a strain to act both as an effective mutualist and a ‘cheater’ depending on the host plant it is inhabiting. This shows that symbiotic effectiveness may be a result of very host-specific interaction at the plant cultivar level while also being strain competition.
Interestingly, all cultivars experienced an increase in SDW when inoculated with a mixture of rhizobia, irrespective of whether the nodule occupancy of effective strains increased. In the context of high strain diversity, it may be possible that plants reward high-performing rhizobia, leading to increased competition between strains and a net benefit to the symbiosis. However, a higher richness of symbiotic partners has been shown to lower symbiotic benefit in mutualisms with Bradyzhizobium (Rahman et al. Citation2023), and has also been linked to an increase in generalist associations in mycorrhizal symbioses (Ramana et al. Citation2023b). The presence of superior strains among mixtures has been shown to be better correlated with host performance rather than total strain diversity in another study (Fields et al. Citation2021). Nevertheless, our results highlight the importance of considering the nuances of competition and resource allocation when examining symbiotic traits (Ramana et al. Citation2023a), as single-strain inoculation may not represent the full breadth of interactions that occur between microbes in root nodules (Shah et al. Citation2021) and therefore may not the best assessment of plant × strain symbiosis. The experiment may also need to run for a longer period of time to see the impact of less effective associations and shoot biomass.
The age of the cultivar, based on when it was released to the market, may have an impact on the ability of a white clover cultivar to form and maintain effective symbiotic partners, and discriminate against, effective and ineffective strains of rhizobia. There was a contrast in nodule occupancy of effective strains between Grasslands Huia (historical) and Grasslands Tribute (modern) (). When presented with a strain mixture inoculant, there was a positive relationship between nodule occupancy and strain effectiveness for Grasslands Huia whereas Grasslands Tribute showed a negative relationship and had a much higher nodule occupancy of its least effective strain. This result indicates that white clover plants may be able to display a degree of partner choice. A previous study using a split root experiment with Medicago truncatula and two strains of Sinorhizobium meliloti displaying different nitrogen fixation phenotypes, showed that the host plant exhibited pre-infection partner choice. The side of the root associated with the N-fixing strain, formed a greater number of nodules than the non-fixing strain (Gubry-Rangin et al. Citation2010). Kiers et al. (Citation2007) showed that older cultivars were more symbiotically competent when infected with a mixture of effective and ineffective rhizobia. Older cultivars produced more seeds in such environments, suggesting their benefit could be a result of partner choice and sanctions against ‘cheater’ strains. This suggested that when infected by symbionts varying in quality, legume defences against poor-quality partners had worsened under artificial selection. Among these three historical and modern pairs of white clover cultivars, the comparison of Grasslands Huia and Grasslands Tribute provides some support for our hypothesis.
White clover is predominantly an outcrossing species and shows disomic inheritance. Therefore, populations of white clover are a heterogeneous mixture of heterozygous individuals leading to high levels of genetic variation both within and between populations. This contributes to broad environmental adaption and phenotypic plasticity of white clover (Woodfield and Caradus Citation1996; Jahufer et al. Citation2013). A study conducted by Jahufer et al. (Citation2013) found significant genotypic variation for a range of aboveground traits such as internode length, node number, stolon branching, and stolon thickness. The five replicates used in our experiments may not have been enough to obtain good statistical resolution and could have been the reason for not seeing more significant differences in the second experiment. However, we were still able to detect some differences in SDW and nodule occupancy of strains. An issue that contributed to the lack of replicates was the low germination of seeds. Due to the age of the seeds, the germination percentage of the historical cultivars was around 30%. These seeds were also expensive and difficult to obtain. This low germination rate made it difficult to obtain enough plants to increase the replication of the experiments. Having seed germination percentage as criteria for cultivar selection may help overcome this issue in the future. All the rhizobia strains used in this study were mined using Grasslands Tribute. This may have introduced a bias where some strains may not be as compatible or competitive in nodulation with historical cultivars. However, in the first experiment the strain forming the most nodules and/or providing the most plant benefit varied among cultivars suggesting our selection of strains provided a good baseline from which cultivars could form effective symbioses.
Conclusions
This is the first study to investigate whether historical cultivars of white clover are able to form more associations with effective strains of rhizobia compared with modern cultivars. The results provide some support to the hypothesis that from a mixture of strains varying in symbiotic efficacy, nodule occupancy of effective strains was greater in historical cultivars compared with modern cultivar of white clover. This study also confirmed reports that different strains of rhizobia vary in their efficacy when in symbiosis with different cultivars. Our results show it is important to consider below-ground mutualisms when selecting cultivars for breeding and highlight the importance of matching adequate symbionts to plant hosts.
Acknowledgements
We thank Celine Blond, and Sandy Hammond for assistance in the lab. Zach Dewhurst provided valuable comments on earlier versions of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akçay E, Simms EL. 2011. Negotiation, sanctions, and context dependency in the legume-rhizobium mutualism. The American Naturalist. 178(1):1–14. doi:10.1086/659997.
- Bates D, Kliegl R, Vasishth S, Baayen H. 2015. Parsimonious mixed models. arXiv preprint arXiv:1506.04967.
- Burdon J, Gibson A, Searle SD, Woods M, Brockwell J. 1999. Variation in the effectiveness of symbiotic associations between native rhizobia and temperate Australian Acacia: within-species interactions. Journal of Applied Ecology. 36(3):398–408. doi:10.1046/j.1365-2664.1999.00409.x.
- Caradus J, Woodfield D, Stewart A. 1995. Overview and vision for white clover. NZGA: Research and Practice Series. 6:1–6.
- Chanway CP, Turkington R, Holl F. 1991. Advances in ecological research. Advances in Ecological Research. 21:121–169. doi:10.1016/S0065-2504(08)60098-7.
- Denison RF. 2021. Legume-imposed selection for more-efficient symbiotic rhizobia. Proceedings of the National Academy of Sciences. 118(22):e2107033118. doi:10.1073/pnas.2107033118.
- Easton H, Amyes J, Cameron N, Green R, Kerr G, Norriss M, Stewart A. 2002. Pasture plant breeding in New Zealand: where to from here? Proceedings of the New Zealand Grassland Association. 64:173–179. doi:10.33584/jnzg.2002.64.2455.
- Fields B, Moffat EK, Friman V-P, Harrison E. 2021. The impact of intra-specific diversity in the rhizobia-legume symbiosis. Microbiology 167(4). doi:10.1099/mic.0.001051.
- Gubry-Rangin C, Garcia M, Béna G. 2010. Partner choice in Medicago Truncatula–Sinorhizobium symbiosis. Proceedings of the Royal Society B: Biological Sciences. 277(1690):1947–1951. doi:10.1098/rspb.2009.2072.
- Irisarri P, Cardozo G, Tartaglia C, Reyno R, Gutiérrez P, Lattanzi FA, Rebuffo M, Monza J. 2019. Selection of competitive and efficient rhizobia strains for white clover. Frontiers in Microbiology. 10:768. doi:10.3389/fmicb.2019.00768.
- Jahufer M, Dunn A, Baird I, Ford J, Griffiths A, Jones C, Woodfield D, Barrett B. 2013. Genotypic variation for morphological traits in a white clover mapping population evaluated across two environments and three years. Crop Science. 53(2):460–472. doi:10.2135/cropsci2012.06.0370.
- Kiers ET, Denison RF. 2008. Sanctions, cooperation, and the stability of plant-rhizosphere mutualisms. Annual Review of Ecology, Evolution, and Systematics. 39:215–236. doi:10.1146/annurev.ecolsys.39.110707.173423.
- Kiers ET, Hutton MG, Denison RF. 2007. Human selection and the relaxation of legume defences against ineffective rhizobia. Proceedings of the Royal Society B: Biological Sciences. 274(1629):3119–3126. doi:10.1098/rspb.2007.1187.
- Kosslak RM, Bohlool BB, Dowdle S, Sadowsky MJ. 1983. Competition of Rhizobium japonicum strains in early stages of soybean nodulation. Applied and environmental Microbiology. 46(4):870–873. doi:10.1128/aem.46.4.870-873.1983.
- Liu A, Ku Y-S, Contador CA, Lam H-M. 2020. The impacts of domestication and agricultural practices on legume nutrient acquisition through symbiosis with rhizobia and arbuscular mycorrhizal fungi. Frontiers in Genetics. 11:583954. doi:10.3389/fgene.2020.583954.
- McKnight T. 1949. Efficiency of isolates of Rhizobium in the cowpea group, with proposed additions to this group: Division of Plant Industry.
- Parker MP. 1995. Plant fitness variation caused by different mutualist genotypes. Ecology. 76(5):1525–1535. doi:10.2307/1938154.
- Rahman A, Manci M, Nadon C, Perez IA, Farsamin WF, Lampe MT, Le TH, Martínez LT, Weisberg AJ, Chang JH. 2023. Competitive interference among rhizobia reduces benefits to hosts. Current Biology. 33(14):2988–3001.e4. e2984. doi:10.1016/j.cub.2023.06.081.
- Ramana JV, Tylianakis JM, Ridgway HJ, Dickie IA. 2023a. Plants select arbuscular mycorrhizal fungi that functionally complement their root traits. Authorea. doi:10.22541/au.169321546.69427297/v1.
- Ramana JV, Tylianakis JM, Ridgway HJ, Dickie IA. 2023b. Root diameter, host specificity and arbuscular mycorrhizal fungal community composition among native and exotic plant species. New Phytologist. 239(1):301–310. doi:10.1111/nph.18911.
- R Core Team R. 2013. R: A language and environment for statistical computing. 275-286.
- Sachs J, Ehinger M, Simms E. 2010. Origins of cheating and loss of symbiosis in wild Bradyrhizobium. Journal of Evolutionary Biology. 23(5):1075–1089. doi:10.1111/j.1420-9101.2010.01980.x.
- Shah A, Wakelin SA, Moot DJ, Blond C, Laugraud A, Ridgway HJ. 2021. Trifolium repens and T. subterraneum modify their nodule microbiome in response to soil pH. Journal of Applied Microbiology. 131(4):1858–1869. doi:10.1111/jam.15050.
- Shi S, Wakelin S, Young GE, van Koten S, Caradus C, Griffiths J, Ballard AG, Callaghan OM. 2023. Screening and field evaluation of white clover rhizobia for New Zealand pastures. Crop & Pasture Science. 74:1258–1271. doi:10.1071/CP22405.
- Simms EL, Taylor DL, Povich J, Shefferson RP, Sachs JL, Urbina M, Tausczik Y. 2006. An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proceedings of the Royal Society B: Biological Sciences. 273(1582):77–81. doi:10.1098/rspb.2005.3292.
- Versalovic J, Koeuth T, Lupski R. 1991. Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial enomes. Nucleic Acids Research. 19(24):6823–6831. doi:10.1093/nar/19.24.6823.
- Wakelin S, Tillard G, van Ham R, Ballard R, Farquharson E, Gerard E, Geurts R, Brown M, Ridgway H, Callaghan OM. 2018. High spatial variation in population size and symbiotic performance of Rhizobium leguminosarum bv. trifolii with white clover in New Zealand pasture soils. PLoS One. 13(2):e0192607. doi:10.1371/journal.pone.0192607.
- Weith SK, Jahufer MZ, Hofmann RW, Anderson CB, Luo D, Ehoche OG, Cousins G, Jones EE, Ballard RA, Griffiths AG. 2022. Quantitative genetic analysis reveals potential to breed for improved white clover growth in symbiosis with nitrogen-fixing Rhizobium bacteria. Frontiers in Plant Science. 13:953400. doi:10.3389/fpls.2022.953400.
- West S, Kiers ET, Pen I, Denison R. 2002. Sanctions and mutualism stability: when should less beneficial mutualists be tolerated? Journal of Evolutionary Biology. 15(5):830–837. doi:10.1046/j.1420-9101.2002.00441.x.
- Westhoek A, Clark LJ, Culbert M, Dalchau N, Griffiths M, Jorrin B, Karunakaran R, Ledermann R, Tkacz A, Webb I. 2021. Conditional sanctioning in a legume– Rhizobium mutualism. Proceedings of the National Academy of Sciences. 118(19):e2025760118. doi:10.1073/pnas.2025760118.
- Woodfield D, Caradus J. 1996. Factors affecting white clover persistence in New Zealand pastures. Proceedings of the New Zealand Grassland Association. 58:229–235. doi:10.33584/jnzg.1996.58.2196.
- Young SD, van Koten C, Gray CW, Cavanagh JAE, Wakelin SA. 2020. Symbiosis between Rhizobium leguminosarum bv. trifolii strain TA1 and a white clover cultivar benefits clover tolerance to cadmium toxicity. New Zealand Journal of Agricultural Research. 63(3):353–364. doi:10.1080/00288233.2019.168039.

