 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Aims
To describe the incidence, aetiology, treatment, and outcomes of farmer-reported clinical mastitis on New Zealand dairy sheep farms.
Methods
A prospective cohort study was conducted on 20 spring-lambing New Zealand sheep milking farms over the 2022–2023 season. Clinical mastitis was defined as a change in the appearance of milk and/or signs of inflammation in the gland. Farmers were required to report all cases of clinical mastitis and collect information on affected ewes’ demographics, clinical features, treatments (where applicable), and outcomes. Milk samples from mastitic glands were submitted for microbiological culture and identification by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF).
Results
Partial or complete clinical mastitis data were available for 236 cases from 221 ewes on 18/20 study farms. Clinical mastitis was diagnosed in 0–6% of ewes at the farm level, with an overall incidence of 1.8 (95% CI = 1.0–3.2)% using the study data, or 2.3 (95% CI = 1.6–3.3)% using the study data and farmer estimates that included unreported cases. Cases occurred mostly in early lactation, with 59% detected during the lambing period (August–October), at a median of 7 (IQR 3, 40) days in milk. The majority of cases featured clots in the milk (59%), swelling (55%), and unevenness (71%) of the glands. Pyrexia (rectal temperature 40.0°C) was diagnosed in 25% of cases and depression (lethargy, inappetence, or inability to stand) in 26% of cases. Treatment was given to 46% of cases, with tylosin being the most commonly used treatment (50% of treated cases). The most common outcome was immediate drying off to be culled without treatment (32%), followed by still milking and recovered but with lasting problems (25%). Nearly half of all the milk samples submitted were culture negative. Streptococcus uberis (14%), non-aureus staphylococci (12%), and Staphylococcus aureus (11%) were the most common isolates, found on 12, 8 and 8 of the 16 farms with microbiological data, respectively.
Conclusions
Clinical mastitis affected up to 6% of ewes at the farm level. Systemic signs were observed in one quarter of affected ewes, suggesting a role for supportive treatment. Clinical mastitis can be severe and challenging to fully resolve in New Zealand dairy sheep.
Clinical relevance
This is the first systematic study of clinical mastitis in New Zealand dairy ewes. It provides baseline information specific to New Zealand conditions for farmers, veterinarians, and other advisors to guide the management of mastitis for the relatively new dairy sheep industry in New Zealand.
Introduction
Sheep milking is a small but growing industry in New Zealand which has not yet amassed as much research as the bovine dairy industry. Limited overseas research is available, but it may not be generalisable to New Zealand farms with their particular breeds, environments, and pastoral and seasonal systems. Mastitis is recognised as one of the most impactful animal health challenges for dairy cows in New Zealand, but we lack information on the impact and management of mastitis on dairy sheep farms. Overseas, mastitis in dairy sheep is known to affect animal wellbeing and mortality, milk quantity, quality, and processing (Jaeggi et al. Citation2003; Leitner et al. Citation2004; Alba et al. Citation2019).
The incidence of clinical mastitis in New Zealand dairy flocks is unclear. French researchers noted in a review that the incidence within a dairy sheep lactation or year was typically less than 5% and comprised largely of sporadic cases, though larger-scale outbreaks had been reported (Bergonier and Berthelot Citation2003). In 1968, a New Zealand cross-sectional survey of non-dairy ewes, in which 19,427 ewes were manually examined in either August–September or January–February, reported signs of clinical mastitis and udder defects in 1.7% of the ewes (Quinlivan Citation1968a).
While the incidence may be low compared to New Zealand dairy cows, (McDougall Citation1998; Petrovski et al. Citation2009), clinical mastitis in dairy sheep can often be severe. Veterinary examinations of mastitic ewes in a Norwegian study of dairy sheep found moderate or severe systemic signs in half of the 509 ewes detected with clinical mastitis, gangrene in 9% of glands, and pyrexia in more than half of affected ewes (Mork et al. Citation2007).
Understanding the aetiology is essential for controlling mastitis. Anecdotally, many New Zealand dairy sheep farmers have conducted individual investigations into mastitis for their own farms, but, to our knowledge, the aetiology has not been systematically studied using a consistent methodology.
Little specific information exists on the treatment of clinical mastitis in sheep. There are no intramammary products registered for lactational or dry ewe therapy in New Zealand. Several injectable antimicrobials are registered for sheep, with only a few stating a milk withholding period, so, in many instances, long milk withholding periods of 35 days (the default withholding period for the off-label use of medicines in New Zealand) are applied, limiting the economic value of treatment due to the prolonged milk discard period.
In view of the above-mentioned lack of published data, the objective of this study was to describe the incidence, aetiology, treatment, and outcomes of farmer-reported clinical mastitis on New Zealand dairy sheep farms.
Materials and methods
All animal manipulations were approved by the Massey University Animal Ethics Committee (application AEC 22/25).
Study design, setting and participants
This was a prospective cohort study conducted on 20 New Zealand commercial sheep milking farms, commencing on each farm’s seasonal start of spring lambing (July–September 2022) and ending when the last ewe was dried off (February–May 2023). The aim was to recruit a mixture of farms (including some who supplied processors and others who processed their own milk) across different regions. Farms were convenience-selected based on these criteria, and also the willingness of farmers to participate and comply with the study procedures. Farmers were contacted through the milk processing companies or directly (independent operators). The 20 study farms were located in the Waikato (n = 12), Wairarapa (n = 2), and Canterbury (n = 6) regions. All farms lambed entirely in the spring except for one farm that sold fresh milk and also had a smaller autumn-lambing flock. All ewes present on the enrolled farms that lambed in the 2022–2023 season were eligible for inclusion in the study.
Study procedures
Farmers were contacted by phone or email approximately fortnightly to December 2022 to provide updates, arrange sample processing, assess protocol compliance, and answer any questions.
On farm procedures
All clinical mastitis diagnoses and procedures were conducted by farm staff. Prior to the start of lambing, the first author met all study farm owners/managers to introduce the study, train them on the definition of clinical mastitis, specimen collection, and storage and record keeping. Farms were provided with a kit that contained forms and a folder for collecting information, specimen jars, paper towels for preparing teats, disposable gloves, marker pens, alcohol-impregnated teat wipes, and two digital thermometers. Farms were given written protocols and summaries of the definition of clinical mastitis, when to look for clinical mastitis, what to do when clinical mastitis was detected, how to collect milk samples aseptically, and a summary of the study protocol (see Supplementary Information 1). In person and online meetings were conducted to provide training in mastitis diagnosis and sample collection to farm managers and staff. A smart phone application was developed for participating farm staff, which included tools for capturing case, treatment, and outcome information, electronic copies of the written study resources, and a video on how to aseptically collect a milk specimen.
Briefly, farm staff were instructed to record information on each clinical mastitis case detected on the farm, collect duplicate milk samples from the affected gland(s), and manage the case according to their usual methods. No case management advice or direction was provided. Farmers were asked to enter treatment (if applicable) and outcome details once the animal’s outcome was known. If these details were not collected, the first author recovered as much information as possible from farm records at in-person interviews at the end of the 2022–2023 season, when farm demographic and management information was also collected, or through email or telephone communications.
Clinical mastitis was defined as a change in the appearance of milk (clots, blood, watery milk, and/or red milk) and/or signs of inflammation in the gland (swelling, uneven udder, pain, lumps, or discharging sores). Events not considered to be clinical mastitis were: subclinical mastitis i.e. a positive rapid mastitis test (defined as any reaction as judged by the farmer) without clinical signs, blood in the milk without signs of inflammation, and conditions limited to the skin, such as warts and parapox virus lesions (orf). Farmers were requested to manually examine all ewes for clinical mastitis at the first time they were handled during the seasonal lambing period (e.g. at lambing or at first milking), by stripping and visually examining milk from both glands, and palpating the udder for inflammation. Farmers were not directed to specifically examine ewes for clinical mastitis at any other time but were asked to report all cases of clinical mastitis that were identified during the usual running of their farms.
For every case of clinical mastitis, staff were required to enter, when known, (1) information about the ewe (identification, age, lambing date, litter size at pregnancy diagnosis, litter size at birth, first milking date, whether it rained on ≥ 2 days in the week before clinical mastitis was detected, whether the ewe lambed indoors or outdoors, and number of ewes that were milked on the date of diagnosis); (2) case details (date of clinical mastitis detection, identification of the affected glands, presence of clots in the milk, colour of the milk, whether a drop in milk yield was noticed in the 2 days prior to diagnosis, whether the gland was painful, swollen, or uneven, whether there were lumps in the gland, whether the gland was gangrenous, the ewe’s rectal temperature, and whether the ewe was depressed); and (3) whether they intended to treat the case. Gangrene was defined as the gland being cold to the touch or sloughing. Depression was defined as lethargy, inappetence, or inability to stand. Staff could also upload photographs and any other files they deemed relevant.
Staff were required to enter treated ewes’ identification, the product(s) used, the dose, frequency, number of treatments, route, and site of administration, milk and meat withholding periods, and any other details or files they deemed relevant.
For all ewes diagnosed with clinical mastitis, staff were required to select one of the following outcomes: (1) still milking: full recovery without lasting effects; (2) still milking: recovered but with lasting effects (e.g. weak gland); (3) dried off: full recovery without lasting effects; (4) dried off: recovered but with lasting effects (e.g. weak gland); (5) culled: due to lasting mastitis problems; (6) culled: due to non-mastitis problems; (7) died: due to mastitis; (8) died: due to non-mastitis problems; or (9) other outcome (describe). Staff were prompted to describe any lasting problems. Dates of drying off, culling, death, or putting out with lambs were required when relevant, and the date of the outcome entry was recorded for all cases.
Information entered via the app was automatically collated into a spreadsheet online and downloaded for analysis, with separate spreadsheets for case, treatment, and outcome information.
Upon diagnosis and prior to any treatment, farm staff collected two milk samples (3–10 mL) from affected glands using an aseptic technique (Supplementary Information 1). As soon as possible after collection, but no later than 3 hours, the samples were stored in the farmers’ freezer. Frozen samples were picked up intermittently and transported on ice to the first author’s laboratory. One sample was shipped on ice to Massey University (Palmerston North, NZ) for microbiology using insulated boxes with ice, packed so the jars were stabilised upright, and kept at −20°C until analysed. The second (duplicate) sample was retained at the first author’s laboratory and kept at −20°C.
Laboratory procedures
The microbiological procedures aimed at identifying bacteria that grow in aerobic conditions. Isolation of Mycoplasma spp. was not attempted. Frozen milk samples were allowed to thaw at room temperature. Thawed samples were swirled and 10 µL of milk was aseptically collected and deposited as a drop on a quarter of a 5% sheep blood agar plate (Fort Richard; Auckland, NZ) and spread using a sterile spreader. Plates were incubated aerobically at 35–37°C for 40–48 hours. After incubation, plates were inspected for bacterial growth and the number of colony types recorded. Plates with three or more colony types were defined as contaminated and not analysed further. For samples with one or two colony types, the number of colonies was recorded for each type. A minimum of one colony type with three or more colonies was necessary for the plate to progress to the next stage, except where a colony morphologically resembled Staphylococcus aureus, when only one colony was required (Gonzalo et al. Citation2019). When all colony types had <3 colonies (and none resembled Staph. aureus), the sample was classified as “no growth”, and no further action was taken. If a colony type had ≥3 colonies (or resembled Staph. aureus), one isolated colony was picked and sub-cultured onto a new 5% sheep blood agar plate and incubated as above to generate an isolate. The isolates were submitted for matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF; Microflex LT Biotyper; Bruker Daltonics, Billerica, MA, USA) for bacterial identification at mEpiLab, Massey University. A protein extraction protocol was used, in which 1 μL of bacteria was added to 300 μL of high-performance liquid chromatography-grade water, mixed in 900 μL of ethanol with a vortex mixer, centrifuged, and the pellet dried.
For cases where the original culture was defined as contaminated, or isolates were not identifiable by MALDI-TOF, the secondary sample was thawed and cultured using the same procedure. In such cases, the result from the secondary sample was used in the analysis.
The bacteriology results were categorised into six groups: (1) growth of Streptococcus uberis; (2) growth of Staph. aureus; (3) growth of non-aureus staphylococci (NAS); (4) growth of “other” bacteria (i.e. Escherichia coli, Arthrobacter gandavensis, enterococci, or Klebsiella spp.); (4) no growth or unidentifiable bacterium; (5) mixed growth (two colony types); and (6) contaminated samples.
Statistical analysis: sample size calculation and data analysis
The number of commercial dairy sheep farms in New Zealand was unknown when this study was developed. It was estimated that at the start of the 2022/2023 milking season there were approximately 40 commercial farms. A target of 20 farms was set. Assuming a whole-season farm-level incidence of clinical mastitis of 1.7% (Queiroga Citation2017), a mean farm size of 750 ewes and 50% of farms having 250–1,500 ewes (SD of 312.5 ewes), and an intraclass correlation coefficient (ICC) of clinical mastitis of 0.04 (Compton et al. Citation2007), a sample size of 255 cases from 20 farms allowed the incidence of clinical mastitis to be estimated with 95% confidence (α = 0.05) and a precision of ±1.25%.
Raw data were collated from the app website (case, treatment, and outcome data), farmer interviews (missing and updated cases, treatment, and outcome data), and microbiology results, and imported into RStudio 4.2.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) for statistical analysis.
The data were collated and merged in wide format by uniquely identifying each case, gland, and ewe on each farm, then examined for completeness, duplications, consistency, and spurious values. Cases occurring within 21 days of a previous case in the same gland, or if the side of the gland in the first or second case was unknown, were assumed to be the same case, and were excluded. Pyrexia was defined as a rectal temperature 40.0°C (Mork et al. Citation2007). Days in milk at mastitis detection was calculated as the absolute difference in days between the lambing date and the date of mastitis detection. Mastitis treatments were categorised based on the active ingredient(s) of each product used. If more than one product was administered concurrently for a case, and only one antibiotic was used, the antibiotic was deemed the primary treatment. If more than one antibiotic was administered in series (none were administered in parallel), the first antibiotic to be administered was deemed the primary treatment. When animals were known to have died but it was unknown if they died or were culled, and for what reason, the outcome was defined as unknown. If microbiology identified two colony types, the MALDI-TOF results were reported for both. When the best and second-best MALDI-TOF matches identified different species for a single colony type, the best match was reported. When ewes had clinical mastitis in both glands and there were MALDI-TOF results available for each gland, both were reported.
The incidence of clinical mastitis was analysed in two ways with different numerators: (1) only the cases for which at least some case, treatment, outcome, and/or microbiology data were collected; and (2) the above cases but also cases that the farmers were aware of but for which no data were collected. For both methods, the denominator was the estimated total number of ewes that entered the milking flock in the 2022–2023 season.
Exploratory data analysis included generating tables of summary statistics and distributional plots, overall and by farm. Relationships between pairs of variables were visualised with frequency tables and plots. Pairwise associations between categorical variables were tested by χ2 analysis unless expected counts were <5, in which case Fisher’s test was used. Unless otherwise stated, all statistical tests were two-tailed, and the critical significance level was set at 5%.
To provide overall estimates of the incidence of clinical mastitis that were adjusted for clustering of clinical mastitis risk within farm, generalised linear regression models were constructed with a random intercept for farm, for both methods of estimating the incidence described above. Both models contained only an intercept. Population-average predicted probabilities (i.e. the marginal predicted probabilities across all farms) and their 95% CI were calculated after adjusting for the bias introduced when converting from the logit to the probability scale, accounting for both model and random intercept standard deviations (Booth and Hobert Citation1998). The ICC, a measure of within-farm correlation (the proportion of variance that is at the farm level), was calculated for clinical mastitis by simulation to adjust for uneven cluster sizes (Goldstein et al. Citation2002). Farm-level incidence was calculated directly from the raw data. Because of the presence of 0% incidence on 4/20 farms, the confidence intervals were calculated using a Bayesian method with a Jeffreys prior of Beta (0.5, 0.5).
To compare the prevalence of clinical signs (clots, pain, swelling, uneven udder, lumps in the udder, gangrene, depression, and fever) separate logistic regression models were constructed to assess the relationship between each clinical sign (dependent variable) and, as separate predictor variables, (a) cases that were pyrexic and non-pyrexic; (b) cases that were depressed and not depressed, and (c) cases that were culture-positive and culture-negative. In all models, farm was included as a fixed effect to account for clustering.
Results
The median peak number of ewes milked per farm at any one time point over the study was 790 (min 171, max 1,530), while the total was approximately 815 (min 171, max 1,530). On two farms, more ewes were brought in when others were dried off, so the total number milked over the season was larger than the peak. All ewes lambed outdoors except on one farm, which lambed all hoggets (1-year-old ewe lambs) and ewes bearing three or more lambs indoors, and on two farms, which brought some ewes indoors during inclement weather (typically ewes bearing three or more lambs).
After removing nine observations deemed to be the same case as a previous observation from the same ewe, partial or complete data were available for a total of 236 cases from 221 ewes on 16 farms. No cases were observed on two farms. On another two farms, clinical mastitis cases were observed but no data were collected for any cases, while on another seven farms the farmers collected at least some data for some but not all cases. Treatment and outcome information was available for 109 and 199 cases respectively. Microbiology results were available for 160 glands from 135 cases from 135 ewes. On one farm, the staff accidentally collected milk samples from both glands of 16 ewes instead of just the affected glands, so up to 16 microbiology samples from non-clinically mastitic glands were included in the microbiology data (it is unclear if the ewes had mastitis in one or both glands).
Partial case information (204/236) arose from cases for which not all information was known by the farmer at the time of mastitis detection (52/236), and cases with at least some case information that were retrospectively added to the dataset at the farmer interviews at the end of the season (152/236). Cases without treatment information (127/236) were recorded as not treated. Fifteen farmers stated that they did not treat all cases, while five stated that they did not treat any cases. Cases without recorded outcomes (37/236) were ewes that were culled or died but the reason was unknown (9/236) or ewes that could not be located at the end of the study (28/236). Missing microbiology data (101/236) arose from recorded cases with at least partial case information but from which no milk samples were collected (10/236), and cases with at least partial case information that were retrospectively added to the dataset (91/236).
Of the 164 cases with lambing location information, 17 (7%) ewes lambed indoors. Reports indicated it had rained on 2 or more days in the week prior to detection for 53/85 (62%) cases with information. The median age at lambing was 3 (min 1, max 8) years for the 219 cases with a known age. Median litter sizes were 2 (min 1, max 4) and 2 (min 1, max 4) lambs at scanning and birth respectively for the 110 and 102 cases with that information.
Incidence and timing of clinical mastitis across the lactation
The data on the incidence of clinical mastitis across the entire lactation are presented using only the cases for which at least some case data were collected either during the study or at the farmer interviews ((A)) and using the same data but adding the cases that were thought or known to have occurred but for which no case data or milk samples were collected ((B)). These extra cases arose from farmer recollection or farm records. Farmers removed lambs from ewes and started milking the ewes within a 10-day period after lambing on 14 farms (six of which removed lambs within 48 hours of birth), while lambs were reared on the ewes for >10 days (4–12 weeks) after parturition on six farms. In the latter group, the first milking (and observation in the milking parlour) was therefore delayed. Those farms are indicated with asterisks in (A and B). After excluding cases occurring within 21 days of the previous case in the same gland (or when one or both glands were not recorded), a second case was recorded in 15 ewes and a third case in one ewe.
Figure 1. Incidence (with 95% CI) of farmer-reported clinical mastitis on 20 dairy New Zealand sheep farms in the 2022–2023 season, (A) based on data collected during the study, and (B) based on estimates made by the farmer at the end of the season (there is no farmer-estimated clinical mastitis incidence for farm L because the number of cases was unknown). Asterisks indicate farms on which the first milking was delayed ≥ 10 days postpartum because lambs were reared on the ewe.
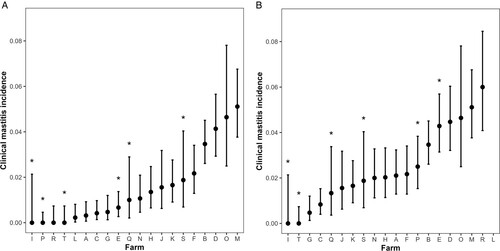
Across all study farms, the overall clinical mastitis incidence over the lactation period was 1.8 (95% CI = 1.0–3.2)%, ranging from 0 to 5.1% at the farm level based on the data collected during the study. The incidence was 0% on four farms, two of which did not collect clinical mastitis data for the study, and the other two diagnosed no cases. For the latter two farms, one of those farms selected milking ewes from a larger conventionally farmed flock at weaning and screened out ewes with any udder defects prior to first milking, unlike the other 19 farms, while the second farm also practiced delayed first milking but had a dedicated milking flock and did not screen ewes. Including cases for which no case data or milk samples were collected, the overall clinical mastitis incidence over the lactation period was 2.3 (95% CI = 1.6–3.3)%, ranging from 0 to 6% at the farm level.
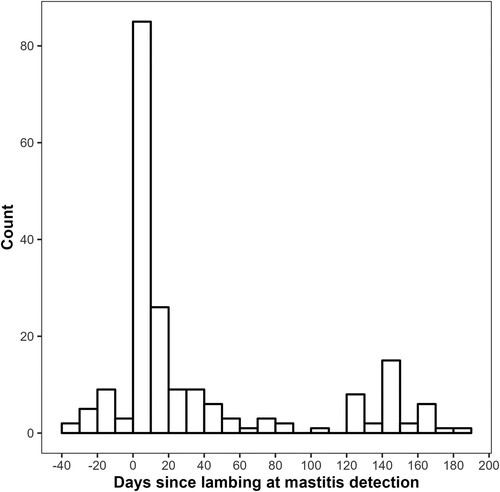
Using only the cases for which study data were collected, clinical mastitis cases were reported between 14 June 2022 and 30 March 2023, with most (139/236; 59%) cases occurring in August to October 2022 (i.e. during the lambing period). Of the cases with known lambing and mastitis detection dates (n = 199), mastitis occurred at a median of 7 (IQR 3–40) days in milk (). Farmers detected 19/199 (10%) of the cases prior to the ewes’ recorded lambing dates. For cases detected on or after the recorded date of lambing (n = 180), the median number of days between lambing and clinical mastitis detection was 11 (IQR 4–46) days.
Figure 2. Timing, in relation to lambing, of farmer-reported clinical mastitis cases on 20 dairy sheep farms in the 2022–2023 season in New Zealand.
Clinical mastitis incidence was not strongly clustered within farms, with ICC of 0.054 using only cases with study data, and 0.016 for cases for which no case data or milk samples were collected.
Clinical features
More cases were detected in the right gland than the left gland and the majority of cases featured clots in the milk, swelling, and unevenness (). The prevalence of some clinical features varied between pyrexic and non-pyrexic ewes, and depressed and non-depressed ewes (). Pyrexia and depression were associated, with 60% (12/20) of depressed ewes being pyrexic, compared to 13% (7/55) of non-depressed ewes. Ewes that were depressed were also more likely to have swollen glands than ewes that were not depressed.
Table 1. Clinical features of farmer-reported clinical mastitis cases (n = 236) on 20 New Zealand dairy sheep farms in the 2022–2023 season.
Table 2. Associations between the presence of pyrexia (rectal temperature 40.0°C) and depression (lethargy, inappetence, or inability to stand) with other clinical features of farmer-reported clinical mastitis cases on 20 New Zealand dairy sheep farms in the 2022–2023 season.
Treatment
Tylosin was the most commonly used primary treatment (). Most injections were administered IM and 49/52 (94%) of IM injections were administered in the rump, while 35/37 (95%) of SC injections were administered in the neck. Meloxicam was used in addition to antibiotics in five cases. For the cases with known milk withholding periods, farmers applied a 3-day milk withholding period for 46/49 (94%) of cases in which tylosin was used, and 35 days for the remaining three cases. Milk withholding periods of 3 (n = 4), 5 (ewes milked twice daily) or 10 (ewes milked once daily) (n = 11), or 35 (n = 10) days were applied for oxytetracycline. The single ewe treated with procaine penicillin had a milk withholding period of 35 days applied. Milk withholding periods of 30 (n = 1) and 35 days (n = 13) were applied to ewes treated with penethamate hydroiodide.
Table 3. Treatment protocols reported by farmers for clinical mastitis cases (n = 109) on 20 New Zealand dairy sheep farms in the 2022–2023 season.
Final case outcomes
Farms varied in their approach to clinical mastitis, but case management was largely applied at the farm level. For example, some farmers frequently attempted treatment, while others instantly dried affected ewes off and placed them in a separate paddock until they could be sent for slaughter, though some ewes were treated for welfare reasons. The most common outcome was instant drying off to be culled without treatment, followed by still milking and recovered with lasting problems (). Only 30/199 (15%) of ewes with outcome information had no lasting problems (and were either still milking at the time the outcome was assessed or had been dried off for other reasons).
Table 4. Reported outcomes among ewes (n = 199) with cases of farmer-diagnosed clinical mastitis (n = 223) on 20 New Zealand dairy sheep farms in the 2022–2023 season.
Of the 21 ewes with an outcome of “other”, eight died or were culled for uncertain reasons, six were instantly dried off without treatment but not culled even though they were not intended to be retained for milking next season, two remained in the flock but it was unknown if they were healthy or if the affected gland had become non-functional (whether or not as a result of the deliberate management actions of the farmer), two had an unknown outcome, one was instantly dried off and was expected to be milked next season, one ewe’s affected gland was no longer functional and the ewe had probably been culled, and one was kept but the udder status was unknown.
When known (n = 67 cases), lasting problems included: the gland became dry or retained only one functional gland (n = 47), the gland became gangrenous, went solid and ruptured, or sloughed (n = 6), the gland milked poorly with or without elevated somatic cell count (n = 5), treatment failure (n = 3), the gland became an aerobic plate count risk (n = 2) where, following microbiological testing by the farmer, the ewe was deemed to be a risk for an elevated bulk milk aerobic plate count, the gland gave a positive milk culture (n = 2), the gland became solid (n = 1), or hard with abdominal swelling in the ewe (n = 1).
Microbiological results
Nearly half (75/160; 46.9%) of all the milk samples submitted were culture-negative (). Secondary milk samples were processed for microbiology for 10 cases due to contamination of the primary sample (n = 6) or having an unidentifiable growth (n = 4). Of the uncontaminated culture-positive samples, only 7/76 (9%) grew 3–10 colonies and 69/76 (91%) grew >10 colonies. Strep. uberis and Staph. aureus were the most common species identified (22/160 and 18/160 respectively), and NAS accounted for 19/160 (12%) of isolates.
Table 5. Aetiology of farmer-diagnosed clinical mastitis cases (n = 160 glands from 135 cases on 16 farms) in a prospective study of clinical mastitis on 20 New Zealand dairy sheep farms in the 2022–2023 season.
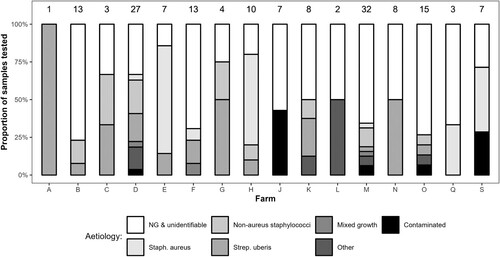
There were clear differences between farms in aetiology, though 5/16 of the farms with bacteriology data had <5 isolates (). Strep. uberis was isolated on 12/16 farms, Staph. aureus on 8/16 farms, NAS on 8/16 farms, and both Staph. aureus and NAS on 2/16 farms. On the 15 farms with microbiology data for more than one case, Strep. uberis was identified in more than one ewe on five farms and Staph. aureus in more than one ewe on three farms.
Figure 3. Microbiological aetiology of farmer-reported clinical mastitis cases (n = 69), excluding contaminated, unidentifiable, and culture-negative (NG) samples, on 20 dairy sheep farms in the 2022–2023 season. The number above the bars represents the number of isolates for each farm. “Other” includes Escherichia coli, Arthrobacter gandavensis, Enterococcus faecalis, and Klebsiella oxytoca.
Swelling was reported in 14/38 (36.8%) and 30/44 (68.2%) culture-negative and culture-positive cases respectively (p = 0.006). The prevalence of other clinical features did not differ significantly between culture-negative and culture-positive cases. For ewes with complete clinical observation data, 23/32 (71.9%) of ewes with both swelling of the affected gland and clots in the milk returned a bacteriologically-positive milk sample, compared to 21/51 (41.2%) of ewes with neither clots nor swelling (p = 0.025).
Discussion
To our knowledge, this is the first systematic study of clinical mastitis on New Zealand dairy sheep farms. We aimed to include all cases of clinical mastitis on the study farms, avoiding the bias that can occur when relying on milk samples collected spontaneously by farmers, who may be more likely to sample certain cases. This study provides evidence-based baseline parameters, collected from about one half of the commercial farms existing at the time of the study. The farms were not randomly selected, but an attempt was made to avoid bias by recruiting both farms supplying large processors and independent operators, across a range of regions.
Nearly all of the ewes with clinical mastitis lambed outdoors, consistent with the management of ewes overall on the study farms. Rain was reported on at least 2 days in the week prior to lambing for most cases, typical of New Zealand’s spring weather. Data were not collected for ewes that did not have a case of clinical mastitis, so no inferences can be made about the effect of lambing indoors, weather conditions, age, and litter size on the risk of clinical mastitis.
Farmer-reported clinical mastitis cases may produce different results to the recording of cases by single investigators, mainly due to a higher risk of deviations from the protocol by the farmers. Farmers and their staff may have made diagnostic or data entry errors, or failed to collect information or milk samples, especially during the extremely busy lambing period (when most clinical mastitis cases occurred), causing the incidence to be underestimated. Unfortunately, it was not logistically feasible to send investigators to record all the cases on 20 geographically dispersed farms. To reduce the amount of error due to non-compliance with the protocol, we provided standardised training to the farm personnel prior to the study and monitored the study periodically. There was a 0.5% absolute difference (a 28% relative difference) in the incidence of clinical mastitis over the lactation period between that estimated from the cases with at least some case information (1.8 (95% CI = 1.0–3.2)%) and the estimate from farmers’ retrospective recollection of cases after the study had finished (2.3 (95% CI = 1.6–3.3)%). However, while the difference between the two estimates is large in relative terms, the estimated incidence of farmer-reported clinical mastitis was low using either method.
The mean clinical mastitis incidence over the lactation period of 1.8 (95% CI = 1.0–3.2)% is consistent with overseas estimates: 0.9% in Portugal (Queiroga Citation2017), and 1.7 cases per 100 ewe-months in Jordan (Lafi et al. Citation1998). However, the three studies differed methodologically. Queiroga (Citation2017) made a point estimate (i.e. a prevalence estimate), which is likely to be lower than the incidence across a season or lactation. Lafi et al. (Citation1998) visited farms monthly, so the incidence may be underestimated. The incidence in the present study was similar to the 1.7% point prevalence estimated by Quinlivan (Citation1968a) in non-dairy New Zealand sheep, but the latter study shares the same limitations as Queiroga (Citation2017). Compared to dairy cows, the clinical mastitis incidence in New Zealand dairy sheep appears to be low, though whether the same is true for subclinical mastitis is unclear. Given the early development stage many dairy sheep farms are at in New Zealand, it is possible that the incidence of clinical mastitis will change over time as the industry evolves due to shifts in management and genetics.
Repeat cases occurring within 21 days were excluded from the analysis if they were in the same gland or the gland in either case was unknown but were included otherwise. However, many ewes were dried off or culled at the first case, so it is not clear what the recurrence rate would be if all ewes were retained, and this should be considered when advising farmers on expected recurrence rates.
The present study was not designed to describe the duration and sequelae of clinical mastitis cases, which would require all ewes to be examined prospectively prior to and after clinical mastitis diagnosis. The prompt removal of ewes observed on many farms in this study meant that duration was often irrelevant. New Zealand researchers examined non-dairy ewes (n = 48) weekly over 6 weeks for udder hardness and lumps and showed that chronic udder changes in ewes can persist for several weeks at least (Zeleke et al. Citation2021).
The between-farm incidence range over the lactation period (0–5%) was relatively small compared to similar work on New Zealand dairy cows. Petrovski et al. (Citation2009) measured the lactation incidence in 16 Northland herds and found an incidence range of 4–33%, and McDougall (Citation1998) found a range of 1–21%. There is very little information on between-farm variation in mastitis incidence for dairy sheep worldwide. Queiroga (Citation2017) reported the point prevalence of clinical mastitis in Portuguese dairy flocks to range from 0 to 8%, which is not very different from our range.
Clinical mastitis was not strongly clustered within farms, with ICC of 0.054 using only cases with study data, and 0.016 cases for which no case data or milk samples were collected. This suggests that efforts to prevent clinical mastitis at the industry level should focus on individual ewes more than farm-level factors, since most of the variance in our study was at the ewe or gland level. We could not locate any published ICC values for clinical mastitis in dairy sheep. Compton et al. (Citation2007) found a similarly low ICC of 0.04 for New Zealand dairy heifers.
Two farms in the present study did not report cases during the study period. One of those farms did not have a dedicated milking flock, and instead chose the desired number of ewes from a larger, conventionally farmed flock on the same property at weaning, and screened out ewes with udder defects. In addition to the absence of mastitis monitoring at milking until weaning, the application of screening prior to entry to the milking flock may have reduced the risk of clinical mastitis by eliminating ewes with mastitis risk factors (that have already caused them to suffer mastitis and develop udder defects), potentially explaining the 0% incidence over the lactation period. The other farm also delayed milking until weaning and therefore may also have missed cases occurring between lambing and first milking. In both cases, it is not possible to determine if clinical mastitis cases were occurring after the first milking but being missed, or if they were both low-incidence flocks due to breeding and management practices. Despite delayed first milking potentially reducing the sensitivity of clinical mastitis detection, there were some farms that commenced milking within 10 days of lambing with a relatively low clinical mastitis incidence, and farms that delayed first milking with a higher incidence ().
Clinical mastitis occurred mostly during lambing and within 14 days of parturition, with some cases seen prior to the recorded lambing date. There is little published information on the timing of clinical mastitis in dairy sheep. In a Norwegian prospective study, 20% of clinical mastitis cases were diagnosed in the first two days of lactation (Mork et al. Citation2007). Under New Zealand conditions, the risk of clinical mastitis is typically highest for dairy cows in early lactation (McDougall Citation1998), which coincides with the often wet spring conditions. It is possible that the intensity of detection, motivation, or awareness of clinical mastitis were higher on the study farms during early lactation, increasing the apparent incidence. Alternatively, mastitis detection may be compromised in early lactation due to the heavy workload typical of seasonal farms’ short and concentrated lambing periods, resulting in underdiagnosis in early lactation. The data presented here suggest that most clinical mastitis occurs in early lactation, during the lambing period, and detection efforts should be maximised over that time.
Visible changes to the udder, such as lumps, swelling, or asymmetry, were common among mastitic ewes. A number of published studies have measured udder defects (Quinlivan Citation1968a; Ridler et al. Citation2021), but fibrosis and abscesses are not necessarily reflective of new cases. Many of the changes in the present study could have been the sequelae of mastitis in previous seasons. Udder defects have been shown to persist across lactations. Zeleke et al. (Citation2023) conducted a longitudinal study, in which the udder halves of 991 non-dairy ewes were assessed four times annually over 2 years. Ewes with udder defects (hardness or lumps) diagnosed at a pre-mating visit had relative risk ratios of having an udder defect later in the same season or at the pre-mating visit in the following season ranging from 6.8 to 1,444, demonstrating the persistence of udder defects.
The lower prevalence of swelling among culture-negative cases suggests that some farmers may be diagnosing chronic udder defects as clinical mastitis, some of which may be the result of previous inflammation. Combining the presence of clots in the milk and swelling of the affected gland did not improve upon swelling alone in terms of predicting the prevalence of culture-negative milk samples. It is therefore challenging to differentiate between active cases of clinical mastitis and sequelae of previous cases on clinical signs alone. Prospective studies recording the presence of deformities at lambing before the majority of clinical mastitis occurs could help to clarify this point. Developing a dry gland (either deliberately by the farmer or not) was the most common lasting problem reported in the present study after a case of clinical mastitis. Many such ewes may present with uneven or otherwise abnormal glands in the following season, and therefore be mistaken as having clinical mastitis.
Negative cultures (“no growth”) was the most common microbiological result in this study, occurring in 75/160 (47%) cases. Mistaking udder defects for clinical mastitis may have contributed to the high proportion of negative cultures, as well as the up to 16 samples collected from non-mastitic glands. Initially, a conservative value of ≥ 10 colonies was considered in the present study as the threshold for a sample to proceed to aetiological diagnosis, to align with previous work on ovine milk microbiology (Fthenakis Citation1994; Lafi et al. Citation1998; Vasileiou et al. Citation2018). However, a more liberal threshold of ≥ 3 colonies was eventually applied, so it appears unlikely that the colony count threshold was responsible for the high prevalence of negative culture results observed in our study. No growth occurred in 13% and 19% of cases in the studies of Mork et al. (Citation2007) and Queiroga (Citation2017) respectively. For non-dairy ewes diagnosed with an udder deformity by Ridler et al. (Citation2021), 47% were culture-negative, using a similar microbiological method.
Another possible explanation for the high prevalence of negative cultures is the freezing and thawing of milk samples. However, data on the effect of freezing on pathogen’s viability in sheep milk are scarce and ambiguous. Smith et al. (Citation2011) compared the recovery rate of bacteria in 50 ewe milk samples known to be infected after freezing for 4 or 8 weeks, with or without preservation with glycerol. The proportion of samples that were bacteriologically positive declined by up to 50% with time across all pathogens regardless of preservation. They found that the lower the CFU count of the sample, the more its viability was impacted by freezing. Samples with CFU counts >100/mL on Day 0 yielded maximum reductions of 25% in isolation after freezing. For culture-negative samples on Day 0, freezing increased the isolation rate for Staph. aureus. Sanchez et al. (Citation2003) demonstrated that freezing milk samples from goats infected with subclinical mastitis at −20°C or −80°C up to 730 days increased the CFU count of coagulase-negative staphylococci (CNS) (Staphylococcus caprae, Staphylococcus epidermidis, Staphylococcus chromogenes and Staphylococcus xylosus) with time and reduced the CFU count of Gram-negative bacilli (Serratia marcescens, Enterobacter cloacae and Pseudomonas aeruginosa) with time when stored at −20°C, but not at −80°C. Schukken et al. (Citation1989) demonstrated that freezing milk samples from cows with clinical or subclinical mastitis at −20°C for up to 16 weeks reduced the number of samples that had cultures of E. coli or Actinomyces pyogenes, increased the number of samples that had cultures of CNS, and had no effect on streptococci and Staph. aureus. In their study of clinical mastitis in dairy ewes, Mork et al. (Citation2007) submitted some samples immediately for culture and froze other samples, but no comparison of the results for fresh and frozen samples was presented. Lafi et al. (Citation1998) and Queiroga (Citation2017) submitted all samples immediately for culture, while Ridler et al. (Citation2021) froze all samples. The limited data from sheep suggest freezing reduces the viability of some bacterial species in milk, though this may be more pronounced in low CFU count samples, and deterioration appears to be greater for Gram-negative pathogens. The samples were collected from ewes diagnosed by farmers as having clinical mastitis, and therefore may have had higher CFU counts than samples from randomly selected healthy ewes or ewes with subclinical mastitis, meaning the effect of freezing may have been less pronounced. However, we cannot rule out an effect of freezing on the high prevalence of no growth samples, particularly in the case of Gram-negative pathogens.
There is little information on the clinical features of sheep mastitis. Mork et al. (Citation2007) reported “moderate or severe” systemic signs in 49% of ewes, but the severity categories were not defined in detail. In the present study, unevenness in the udder was the most common observation (71% of cases). Depression and pyrexia occurred in 26 and 25% of cases respectively. Mork et al. (Citation2007) diagnosed pyrexia in 56% of cases using the same definition (i.e. ≥ 40.0°C) in our study. We observed gangrene in 6.5% of ewes, similar to the 8.8% reported by Mork et al. (Citation2007). The frequent reports of pyrexia, depression, and gangrene indicate that, while clinical mastitis in dairy ewes may be less common than is typical for dairy cows under New Zealand conditions, it can often be severe and should be addressed promptly for animal welfare and productivity reasons. Depression is a somewhat subjective measure but appears to be associated with the severity of the mastitis and may be a useful observation for farmers to use.
Tylosin was the most common treatment (50% of treated cases) recorded in our study. It is unclear if this is due to a genuine or perceived high efficacy or favourable registration conditions compared to other products. Many farmers however, did not treat ewes with clinical mastitis at all, with only 109/236 (46%) cases recorded as receiving treatment. The use of anti-inflammatory drugs (meloxicam) was only reported in five cases. The 25% prevalence of pyrexia, and the association between pyrexia and depression, suggest that treatment with non-steroidal anti-inflammatory drugs could be a useful part of a dairy ewe clinical mastitis treatment toolkit, especially on farms where such ewes are not immediately culled.
Full recovery without lasting problems was only reported for 8.5% of the ewes. The most common problem (when recorded) was developing a dry gland or being deliberately made into a ewe with only one functional gland. Even those ewes with an outcome of “still milking (full recovery)” (i.e. no lasting problems) may have deteriorated subsequently, meaning the probability of a full recovery was even lower. The likelihood of recovering without lasting problems is unclear for dairy cows, but clinical failure (as defined by the farmer) among New Zealand dairy cows diagnosed with clinical mastitis due to a range of pathogens was 13.4% when treated with an intramammary product containing amoxicillin, clavulanic acid, and prednisolone twice daily for five treatments (McDougall et al. Citation2019). Other authors have discussed the low probability of complete recovery and the objective of treatment being to avoid death and cull the ewe (Bergonier and Berthelot Citation2003). In a review of mastitis in sheep, Watson and Buswell (Citation1984) commented that “The course of the disease is rapid and its effects severe, so much so that treatment is invariably directed at saving the life of the ewe in the full knowledge that changes to her affected udder tissue are irreversible”. It is not possible to draw inferences between outcomes and clinical presentation or treatment choice because treatment and management were largely applied at the farm level (e.g. a farm used only one treatment, or all mastitic ewes were dried off instantly without treatment). When developing treatment plans, veterinarians should set realistic expectations for the probability of ewes making full recoveries from an udder health and productivity perspective and remain conscious of protecting the ewe’s welfare while attempting treatment.
Overall, the aetiology was predominantly Gram-positive, with Strep. uberis, Staph. aureus, and NAS the most common isolates. Staphylococci have been repeatedly shown as the most common cause of clinical mastitis in dairy sheep overseas (Bergonier and Berthelot Citation2003; Mork et al. Citation2007; Queiroga Citation2017) and were the most common bacteria isolated from ewes with udder defects among non-dairy sheep in New Zealand (Quinlivan Citation1968b; Ridler et al. Citation2021). However, S. aureus and NAS were only isolated on 50% and 44% of farms that submitted milk samples for culture, suggesting some clustering of aetiology by farm. In contrast, Strep. uberis appeared more frequently than in previous work from overseas. It accounted for 14.0% of cases in the present study, while it accounted for 1.6% of cases in the study of Mork et al. (Citation2007), was not found in the studies of Queiroga (Citation2017) or Lafi et al. (Citation1998), and only accounted for 1.9% of samples from non-dairy ewes with udder defects in the study of Ridler et al. (Citation2021). A higher contribution by Strep. uberis to clinical mastitis for New Zealand dairy ewes, compared to New Zealand non-dairy ewes and dairy ewes in other countries, may be a feature of our predominantly outdoor, spring-lambing systems. Strep. uberis is recognised to be a common cause of clinical mastitis in New Zealand dairy cows, which, like the dairy sheep in this study, predominantly graze outdoors all year. Petrovski et al. (Citation2009) isolated Strep. uberis from 23.3% of cases of clinical mastitis in a study conducted on 14 New Zealand dairy farms milking a total of 3,765 cows.
Conclusions
Clinical mastitis affected 0–6% of ewes at the farm level across the 2022–2023 season, with an overall lactation incidence of 1.8 (95% CI = 1.0–3.2)% using the study data, or 2.3 (95% CI = 1.6–3.3)% using study data and farmer estimates. However, 46.9% of cases were culture-negative, and culture-negative cases were less likely to have swelling of the gland, suggesting the incidence might have been overestimated. Cases occurred predominantly over the lambing period and within the first 14 days of lactation. Pyrexia and depression were reported in 25 and 26% of ewes respectively, suggesting a role of supportive treatment for systemic signs of clinical mastitis. Clots in the milk and swelling and unevenness of the glands were recorded in the majority of cases. Pyrexia and swelling were more likely among ewes that were clinically depressed, and clots and swelling were more prevalent among glands that returned culture-positive milk samples. Most ewes did not recover without lasting problems such as a dry gland. Strep. uberis (14%), non-aureus staphylococci (12%), and Staph. aureus (14, 12, and 11% of cases with microbiology data) were the most common isolates.
Supplemental Material
Download PDF (874.9 KB)Acknowledgements
Funding for this study was provided by AGMARDT, Boehringer Ingelheim, EpiVets, Massey University, Maui Sheep Milk, MilkTestNZ, The New Zealand Veterinary Association, Sheep Milk New Zealand, Spring Sheep, and Virbac.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alba DF, da Rosa G, Hanauer D, Saldanha TF, Souza CF, Baldissera MD, Dos Santos DDS, Piovezan AP, Girardini LK, Schafer ADS. Subclinical mastitis in Lacaune sheep: Causative agents, impacts on milk production, milk quality, oxidative profiles and treatment efficacy of ceftiofur. Microbial Pathogenesis 137, 103732, 2019. https://doi.org/10.1016/j.micpath.2019.103732
- Bergonier D, Berthelot X. New advances in epizootiology and control of ewe mastitis. Livestock Production Science 79, 1–16, 2003. https://doi.org/10.1016/S0301-6226(02)00145-8
- Booth JG, Hobert JP. Standard errors of prediction in generalized linear mixed models. Journal of the American Statistical Association 93, 262–272, 1998. https://doi.org/10.1080/01621459.1998.10474107
- Compton CW, Heuer C, Parker K, McDougall S. Epidemiology of mastitis in pasture-grazed peripartum dairy heifers and its effects on productivity. Journal of Dairy Science 90, 4157–70, 2007. https://doi.org/10.3168/jds.2006-880
- Fthenakis GC. Prevalence and aetiology of subclinical mastitis in ewes of Southern Greece. Small Ruminant Research 13, 293–300, 1994. https://doi.org/10.1016/0921-4488(94)90078-7
- Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models. Understanding Statistics 1, 223–231, 2002. https://doi.org/10.1207/S15328031US0104_02
- Gonzalo C, Juárez MT, García-Jimeno MC, De La Fuente LF. Bulk tank somatic cell count and total bacterial count are affected by target practices and milking machine features in dairy sheep flocks in Castilla y León region, Spain. Small Ruminant Research 178, 22–29, 2019. https://doi.org/10.1016/j.smallrumres.2019.07.007
- Jaeggi JJ, Govindasamy-Lucey S, Berger YM, Johnson ME, McKusick BC, Thomas DL, Wendorff WL. Hard ewe’s milk cheese manufactured from milk of three different groups of somatic cell counts. Journal of Dairy Science 86, 3082–9, 2003. https://doi.org/10.3168/jds.S0022-0302(03)73908-3
- Lafi SQ, al-Majali AM, Rousan MD, Alawneh JM. Epidemiological studies of clinical and subclinical ovine mastitis in Awassi sheep in northern Jordan. Preventive Veterinary Medicine 33, 171–81, 1998. https://doi.org/10.1016/S0167-5877(97)00048-2
- Leitner G, Chaffer M, Shamay A, Shapiro F, Merin U, Ezra E, Saran A, Silanikove N. Changes in milk composition as affected by subclinical mastitis in sheep. Journal of Dairy Science 87, 46–52, 2004. https://doi.org/10.3168/jds.S0022-0302(04)73140-9
- McDougall S. Prevalence of clinical mastitis in 38 Waikato dairy herds. Proceedings of the New Zealand Society of Animal Production 58, 76–8, 1998
- McDougall S, Clausen L, Hintukainen J, Hunnam J. Randomized, controlled, superiority study of extended duration of therapy with an intramammary antibiotic for treatment of clinical mastitis. Journal of Dairy Science 102, 4376–4386, 2019. https://doi.org/10.3168/jds.2018-15141
- Mork T, Waage S, Tollersrud T, Kvitle B, Sviland S. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features. Acta Veterinaria Scandinavica 49, 23, 2007. https://doi.org/10.1186/1751-0147-49-23
- Petrovski KR, Heuer C, Parkinson TJ, Williamson NB. The incidence and aetiology of clinical bovine mastitis on 14 farms in Northland, New Zealand. New Zealand Veterinary Journal 57, 109–115, 2009. https://doi.org/10.1080/00480169.2009.36887
- Queiroga MC. Prevalence and aetiology of sheep mastitis in Alentejo region of Portugal. Small Ruminant Research 153, 123–130, 2017. https://doi.org/10.1016/j.smallrumres.2017.06.003
- Quinlivan TD. Survey observations on ovine mastitis in New Zealand stud Romney flocks. 1. The incidence of ovine mastitis. New Zealand Veterinary Journal 16, 149–53, 1968a. https://doi.org/10.1080/00480169.1968.33765
- Quinlivan TD. Survey observations on ovine mastitis in New Zealand stud Romney flocks. 2. The bacteriology of ovine mastitis. New Zealand Veterinary Journal 16, 153–60, 1968b. https://doi.org/10.1080/00480169.1968.33766
- Ridler AL, Rout-Brown G, Flay KJ, Velathanthiri N, Grinberg A. Defects and bacterial pathogens in udders of non-dairy breed ewes from New Zealand. New Zealand Journal of Agricultural Research 65, 163–171, 2021. https://doi.org/10.1080/00288233.2021.1905005
- Sanchez A, Contreras A, Jimenez J, Luengo C, Corrales JC, Fernandez C. Effect of freezing goat milk samples on recovery of intramammary bacterial pathogens. Veterinary Microbiology 94, 71–7, 2003. https://doi.org/10.1016/S0378-1135(03)00066-X
- Schukken YH, Grommers FJ, Smit JA, Vandegeer D, Brand A. Effect of freezing on bacteriologic culturing of mastitis milk samples. Journal of Dairy Science 72, 1900–1906, 1989. https://doi.org/10.3168/jds.S0022-0302(89)79309-7
- Smith EM, Monaghan EM, Huntley SJ, Green LE. Preliminary investigation into the effect of freezing and a cryopreservant on the recovery of mastitis pathogens from ewe milk. Journal of Dairy Science 94, 4850–5, 2011. https://doi.org/10.3168/jds.2010-4076
- Vasileiou NGC, Cripps PJ, Ioannidi KS, Chatzopoulos DC, Gougoulis DA, Sarrou S, Orfanou DC, Politis AP, Gonzalez-Valerio TC, Argyros S, et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. Journal of Dairy Science 101, 7297–7310, 2018. https://doi.org/10.3168/jds.2017-14075
- Watson DJ, Buswell JF. Modern aspects of sheep mastitis. British Veterinary Journal 140, 529–34, 1984. https://doi.org/10.1016/0007-1935(84)90003-4
- Zeleke MM, Kenyon PR, Flay KJ, Aberdein D, Pain SJ, Peterson SW, Ridler AL. Effect of palpable udder defects on milk yield, somatic cell count, and milk composition in non-dairy ewes. Animals 11, 2831, 2021. https://doi.org/10.3390/ani11102831
- Zeleke MM, Flay KJ, Kenyon PR, Aberdein D, Pain SJ, Ridler AL. Assessment of changes in udder half defects over time in non-dairy ewes. Animals 13, 784, 2023. https://doi.org/10.3390/ani13050784

