ABSTRACT
Many cavity nesters add volatile plants with medicinal properties to their nests. In Australia, eucalyptus and tea-trees are highly volatile plants, which have antiparasitic and antimicrobial properties. Here, we tested whether scents of eucalypt (Eucalyptus polybractea) and tea tree (Melaleuca alternifolia) essential oils can attract birds to newly installed nest boxes. Twenty-four nest boxes received three scent treatments (eucalypt, tea tree, control) for 10 days each using a Latin-square design. Bird visitation was recorded using camera traps. Eastern Rosellas (Platycercus eximius) visited the nest boxes the most (1,454 visits) and were therefore the focus of the study. Eastern Rosellas did not change their behaviours in response to the eucalypt scent in nest boxes: there was no difference in visitation, duration of visits or nest box inspection behaviour between nest boxes with eucalypt scent and those with the control scent. However, Eastern Rosellas visited nest boxes with tea tree scent less frequently and spent less time at these boxes than those with the control scent. The mean time that Eastern Rosellas spent inspecting boxes slightly increased over the 10-day period following scent placement at boxes with control and eucalypt scents, while it sharply decreased at boxes with tea tree scent. This is the first study demonstrating that an Australian bird detected and behaved differently in response to plant volatiles. The use of scents may offer opportunities to not only attract, but potentially also deter certain birds from using particular hollows.
Introduction
Nests are critical for successful reproduction in the majority of birds. They provide protection to fragile eggs, nestlings and brooding parents from temperature fluctuations, extreme weather events and predators (Hansell Citation2000). Nests generally consist of a structural element such as sticks and woven grasses, and a lining of materials such as moss, animal hair or feathers for warmth and comfort (Hansell Citation2000). Additionally, several bird species add fresh green plant material to their nests after nest building has been completed (Scott-Baumann and Morgan Citation2015). This plant material consists of fresh green leaves, flowers, stems or sprigs (Dubiec et al. Citation2013). The plants added are often highly volatile giving the nest environment an aromatic quality. For example, birds include aromatic plants such as yarrow (Achillea spp.), lavender (Lavandula spp.) and wormwood (Artemisia spp.) (Clark and Mason Citation1985; Gwinner Citation1997; Lambrechts and Dos Santos Citation2000; Veiga et al. Citation2006; Møller et al. Citation2013). These fresh green plants are selected non-randomly by birds and incorporated in much larger proportions than they occur in the environment (Scott-Baumann and Morgan Citation2015). Collecting aromatic plants requires considerable investment by birds at a critical time in the breeding cycle, which has led scientists to hypothesise why birds are engaging in this behaviour.
The ‘nest protection hypothesis’ states that birds incorporate volatile plants with phytochemicals (chemicals produced by plants) into their nests to reduce the negative effects of ectoparasites (parasites that live on the external surface of the host, e.g. fleas and mites) and microbes in the nest environment (Clark and Mason Citation1985). This may be of particular benefit to birds that re-use nests over several breeding attempts because the accumulation of excrement and old nesting materials from multiple breeding seasons can lead to an increase in harmful bacteria and ectoparasites, which can decrease the health and survival of chicks (Merino and Potti Citation1995; Zabłotni et al. Citation2020). Indeed, cavity nesting species that often re-use the same hollows during multiple breeding attempts, are particularly well known for this behaviour (Clark and Mason Citation1987). Several studies have shown that aromatic plants in nests benefit the health of chicks and reduce ectoparasite abundance in nests (Clark and Mason Citation1988; Gwinner et al. Citation2000, Citation2018; Mennerat et al. Citation2008, Citation2009; Tomás et al. Citation2012; Pires et al. Citation2020; Yang et al. Citation2020).
Ninety-two per cent of Australian plants are endemic and many of them have highly aromatic volatiles that are renowned for their antibacterial properties (Chapman Citation2009). However, very few studies have investigated which aromatic plants are added by Australian birds in their nests (but see Metcalf et al. Citation1989; Gibbons and Lindenmayer Citation2002; McGuire and Kleindorfer Citation2007) and only one previous study examined the adaptive value of Australian birds adding those volatile plants to their nests (Potvin et al. Citation2021). That study inspected museum collections of Australian birds’ nests and found that increased incorporation of fresh green plant material into nests correlated to a decrease in the number of dead ectoparasite larvae. However, Potvin et al. (Citation2021) did not specify which bird species the nests belonged to or what plant species were used. Furthermore, it remains unknown whether some Australian birds can detect the odours of plant species incorporated into their nests, as overseas experimental studies have shown to be case for Eurasian Blue Tits (Cyanistes caeruleus), Common Starlings (Sturnus vulgaris) and Russet Sparrows (Passer cinnamomeus) (Petit et al. Citation2002; De Groof et al. Citation2010; Yang et al. Citation2020). These studies suggested that olfaction is used during the selection of fresh green material and to maintain the aromatic environment of the nest, however, few experimental studies have been conducted to explicitly test whether birds can detect these plants by smell (Clark and Mason Citation1987; Clark and Smeraski Citation1990; Petit et al. Citation2002).
In Australia, bush fires, climate change, and large-scale logging of old, mature trees have severely limited nesting opportunities for cavity-nesting birds (Gibbons and Lindenmayer Citation2002; Lindenmayer et al. Citation2014; Hunter Citation2015). As a result, many populations of hollow-nesting birds, such as Gang-gang Cockatoos (Callocephalon fimbriatum), Turquoise Parrots (Neophema pulchella), and Brown Treecreepers (Climacteris picumnus) have been declining throughout Australia (Gibbons and Lindenmayer Citation2002; Manning et al. Citation2013). To mitigate the shortage of naturally occurring tree hollows, land managers commonly instal nest boxes (Callan et al. Citation2023). The addition of plant volatiles to nest boxes may be a simple method for attracting birds to nest boxes. Eucalypts (Eucalyptus spp.) and tea trees (Melaleuca and Leptospermum spp.) are common plants which produce volatiles that have antibacterial and antiparasitic properties (Kim et al. Citation2004; Puvača et al. Citation2019) and the incorporation of these plants in nests could potentially be beneficial for cavity nesting birds. Here, we investigated whether Australian cavity-nesting birds respond to the smell of essential oils produced from blue mallee (Eucalyptus polybractea) and narrow leaved paperbark (Melaleuca alternifolia) inside nest boxes. We hypothesised that hollow nesting birds that are inspecting newly installed nest boxes during the pre-breeding season will be attracted to those boxes containing eucalyptus or tea tree scents by a) visiting scented nest boxes more often than control boxes and b) spending more time at scented nest boxes than control boxes.
Methods
Study area
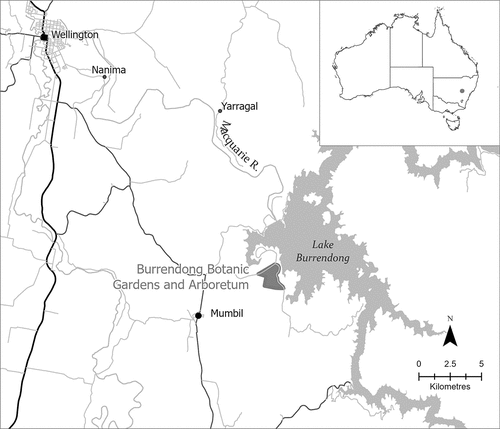
This study was conducted in the Burrendong Botanic Garden and Arboretum (32° 42′ 23″ S, 149° 06′ 30″ E), a 164 ha private reserve in New South Wales situated 25 km south-east of the township of Wellington and 330 km south-west of Sydney ().
Figure 1. Location of the Burrendong Botanic Garden and Arboretum, near Wellington, NSW, where the study on the effects of plant scent on nest box visitation by Eastern Rosellas was conducted.
Twenty-five HabitechTM nest boxes were installed in April 2022 in an area of open White Box (Eucalyptus albens) woodland in the arboretum. As nest boxes were newly installed at this site, past use of nest boxes or breeding success did not influence visitation to those boxes. The HabitechTM nest boxes have plastic outer walls, plywood internal walls and Eucalyptus mulch present in the bottom of the box (Supplementary material; Figure S1; Habitat Innovation and Management Citationn.d.). All nest boxes were in a semi-circle design (see Figure S1) with the same dimensions (width = 28 cm, depth = 14 cm, length = 62 cm); however, the entrance holes varied in size (between 6 and 9 cm in diameter) and location (top entrance and side entrance, Figure S1). Nest boxes were installed at different heights above ground (mean = 175 ± 43.67 cm) and aspects (mean = 172° ± 91.88°). Five days before the experiment began on 16/7/22 the nest boxes were checked with a nest box camera for resident animals to reduce sampling bias and comply with animal ethics requirements. One nest box was excluded from this study due to permanent residence of a Common Brushtail Possum (Trichosurus vulpecula), leaving 24 nest boxes in the experiment. A Reconyx HC600 motion-triggered camera was installed ~1.2 m above each nest box to capture birds entering and exiting nest boxes. When triggered by movement, cameras captured 10 photos at 1-s intervals (key settings used; trigger: sensitive, night mode: max range, quiet period: no delay).
Scent experiment
The experiment was conducted in the period from 16/7/22 to 18/8/22 during the pre-breeding season, when birds are actively searching for cavities but not nesting within them. To account for variation in the placement of the 24 nest boxes, we used an experimental design in which each nest box was sequentially assigned all the scented and unscented treatments over 10-day periods. Order of treatment was randomly assigned so that an equal number of nest boxes (8) was in each treatment group (supplementary material; Table S1). Nest boxes were treated by adding a filter paper soaked with 1 ml of plant volatile (essential oils of either Eucalyptus polybractea or Melaleuca alternifolia), while control boxes received a filter paper prepared with 1 ml of distilled water. Filter papers were screwed into the same position on the right inner wooden plank of each nest box. To avoid cross contamination of scents across nest boxes and trials, all filter papers had a plastic sheet backing to minimise any scents transferring onto the nest boxes. We used clean, disposable, unscented gloves, every time filter paper was handled. All nest boxes were monitored for 10 days using the motion-triggered cameras starting the day after treatment was added. After 10 days, we returned to all nest boxes to remove previous filter papers and add the next experimental treatment. When filter papers from previous treatments were removed from nest boxes, the inside of each nest box was cleansed with distilled water and a fresh paper towel before the next scent was installed. The day after scents were changed, all nest boxes were monitored for further 10 days before we once again changed the treatment at each nest box. The experiment ended after 30 days of observation when all cameras and filter papers were removed from nest boxes.
Study species
Four species visited the nest boxes. We recorded 1454 visits by Eastern Rosella (Platycercus eximius), 86 visits by Brushtail Possum, 30 visits by Feather-tailed Glider (Acrobates spp.) and 30 visits by Glider spp. (Krefft’s Glider, Petaurus notatus or Squirrel Glider, Petaurus norfolcensis). Data analysis was conducted only on Eastern Rosellas, which were by far the species that visited nest boxes the most often. As it was unknown what species may visit the newly installed nest boxes, birds were not uniquely banded before our scent experiment. Hence, we were unable to identify whether the same individuals visited one or more of our nest boxes during our experiment.
Data collection and processing
Photos taken by the motion-triggered cameras were processed by CPW Photo Warehouse version 4.3.0.6 (Newkirk Citation2016). A visit was defined as an event where an Eastern Rosella was photographed at the entrance of a nest box. A visit began when a camera was triggered by an Eastern Rosella at the entrance of the nest box and ended when the bird left the entrance of the nest box. If cameras were triggered within 10 s of the last photo set, then it was assumed that it was the same visit by the same individual. However, if the bird from the first visit was clearly photographed leaving the nest box prior to returning, or another bird was photographed at the nest box, we considered this as two separate visits. For each visit, we recorded the time in seconds which the bird visited and the time in seconds which the visiting bird spent with its head inside the entrance hole inspecting the nest box which we termed inspection behaviour. From these data, we obtained the following response variables: the number of visits to each nest box per day, the sum and the mean duration of visits per nest box per day, and the sum and mean duration of nest box inspection behaviour per nest box per day.
Data analysis
Data analyses were conducted in R studio (v. 4.2.2, R Core Team Citation2022). We used generalised linear mixed models (GLMMs) because they are a robust tool to analyse non-normal data and allow for the inclusion of random effects (Harrison et al. Citation2018). GLMMs modelled each response variable against three fixed factors: treatment (smell treatment present in nest box), order (the order of treatments: order 1 = first 10 days of experiment, order 2 = second 10 days of experiment and order 3 = third and last 10 days of experiment) and treatment day within each treatment order (days since commencement of treatment). Treatment day was considered continuous. An interaction between treatment and treatment day (treatment × treatment day) was considered because volatility (smell) of eucalypt and tea tree treatment decreases with increasing treatment day (Bolker et al. Citation2009). If the interaction’s partial p value did not contribute to the model, because it was not significant, it was omitted. Nest box number was included as a random effect in all models to account for repeat visits to favourable sites.
As our sampling unit was visits per day per nest box, our sample size was n = 720 (30 days * 24 nest boxes). The number of visits per day per nest box (visitation) was zero inflated due to multiple days when birds did not visit nest boxes. We tested the visitation with different distribution families (Poisson and Negative binomial) and several zero influence factors (see supplementary material). Duration responses included total time spent at nest boxes per visit (total duration) and mean time spent at nest box per visit (mean duration). These data were modified to remove days when there were no visits. Total and mean duration data were transformed by ln(x + 1) and analysed using a Gaussian (log) family GLMM. Nest box inspection models were analysed using a two-step method (Boulton and Williford Citation2018). Days were labelled 1 when nest box inspection occurred and 0 when no nest box inspection occurred during visits. The first step was to analyse the data in terms of presence/absence of nest box inspection. These data were put into a binomial (logit) GLMM to assess which fixed factors affected the presence of nest box inspection. The second step was to reduce the data down to only the data where nest box inspection is present and analyses the effects of fixed factors when nest box inspection does occur. Nest box inspection response variables included total time spent inspecting the nest box per visit and mean time spent inspecting the nest box per visit. Total and mean nest box inspection time data were transformed with a ln(x + 1) and analysed using a Gaussian (log) family GLMM.
All models had their residuals tested and simulated with ‘DHARMa’ to determine model applicability (Hartig Citation2017). If models had good residual plots, they were compared using Akaike Information Criteria (AIC) and AIC weights (Symonds and Moussalli Citation2011). If there were several models with low delta AICs, the nested rule was used to limit the models considered (Harrison et al. Citation2018). Package ‘lme4’ (Bates et al. Citation2015) and ‘glmmTMB’ (Brooks et al. Citation2017) were used to create the GLMMs. Laplace approximation was used to estimate the maximum likelihood for the models (Bolker et al. Citation2009). Figures were made by using the predicted mean value of the models using ‘ggemmeans’ (Lüdecke et al. Citation2020). The log transformed data were back-transformed for plotting.
Results
Visits
Eastern Rosellas visited 23 out of 24 nest boxes. The mean number of visits per nest box by Eastern Rosellas (excluding the nest box that was not visited at all) was 63.21 ± 14.53 visits. The mean number of visits per nest box per day was 2.02 ± 0.80 visits.
There was no significant effect of eucalypt treatment on visits compared to the control treatment. Eastern Rosellas visited nest boxes with tea tree treatment significantly less than the control nest boxes (estimate = −0.323 ± 0.142, p = 0.023; , ). In the first 10 days of the experiment (order 1), there was a higher likelihood of days with no visit to nest boxes by Eastern Rosellas compared to the third 10 days of the experiment (order 3 estimate = −5.581 ± 2.737, p = 0.041, ). As treatment day increased, the likelihood of days with no visits decreased (Estimate = −0.637 ± 0.260, p = 0.014, ).
Figure 2. Effects of plant scent treatments on the predicted mean number of visits per day per nest box by Eastern Rosellas. Error bars indicated 95% confidence intervals. Significance levels: p < 0.05 ‘*’.
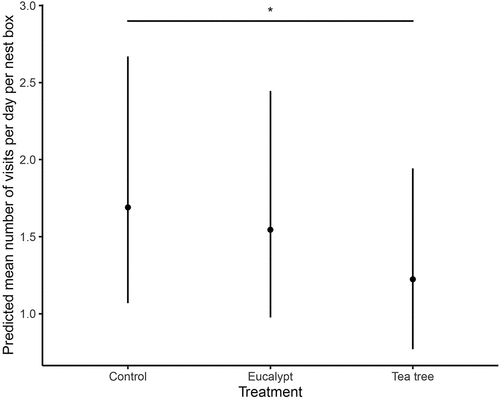
Table 1. Results of a zero-influenced, negative binomial (nbinom 1) (log) GLMM testing whether the number of visits to each nest box per day by Eastern Rosellas was significantly influenced by a plant volatile inside boxes (eucalyptus, tree tree, distilled water), treatment order (order 1, 2 and 3 representing the first, second and last 10 days of the experiment, respectively) or treatment day within each order. The effect of eucalyptus and tea tree treatments were determined by comparing them to the effects of the distilled water. Similarly, the effects of treatment order 2 and order 3 were determined by comparing them to the effects of order 1. Significance levels: p < 0.001 ‘***’, p < 0.01 ‘**’, p < 0.05 ‘*’.
Duration
The total time Eastern Rosellas spent at the nest boxes over the study period was 517.8 min, with a mean of 104.6 ± 9.01 s per visit.
There were no significant effects of eucalypt on the duration of visits. Eastern Rosellas spent significantly less total time (estimate = −0.332 ± 0.152, p = 0.030, , ) and mean time (estimate = −0.200 ± 0.097, p = 0.040, , ) at nest boxes with tea tree treatment compared to the control. The mean time spent at the nest box per visit was significantly lower in the last 10 days of the experiment (order 3; estimate = −0.244 ± 0.101, p = 0.016) than in the first 10 days of the experiment (order 1, , Supplementary material Figure S2).
Figure 3. Effects of plant scent treatments on (a) the predicted total time spent at each nest box, and (b) the predicted mean time spent at each nest box per visit by Eastern Rosellas. Error bars indicate 95% confidence intervals. Significance levels: p < 0.05 ‘*’.
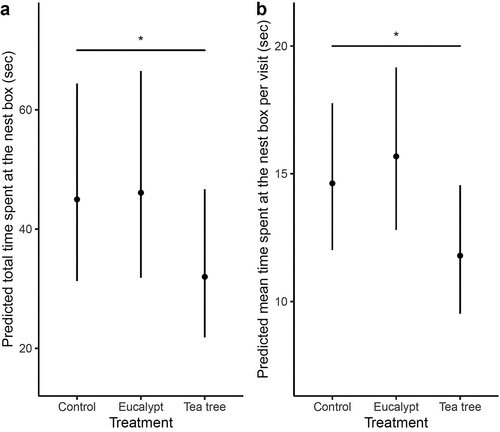
Table 2. Results of a Gaussian GLMM testing whether the total time (ln(x + 1) transformed) spent at each nest box per visit by Eastern Rosellas was significantly influenced by a plant volatile inside boxes (eucalyptus, tree tree, distilled water), treatment order (order 1, 2 and 3 representing the first, second and last 10 days of the experiment, respectively) or treatment day within each order. Significance levels: p < 0.001 ‘***’, p < 0.01 ‘**’, p < 0.05 ‘*’.
Table 3. Results of a Gaussian GLMM testing whether the mean time (ln(x + 1) transformed) spent at each nest box per visit by Eastern Rosellas was significantly influenced by plant volatile inside boxes (eucalyptus, tree tree, distilled water), treatment order (order 1, 2 and 3 representing the first, second and last 10 days of the experiment, respectively) or treatment day within each order. Significance levels: p < 0.001 ‘***’, p < 0.01 ‘**’, p < 0.05 ‘*’.
Table 4. Results of a Gaussian GLMM testing whether the mean time (ln(x + 1) transformed) inspecting each nest box from the entrance hole per visit by Eastern Rosellas was significantly influenced by a plant volatile inside boxes (eucalyptus, tree tree, distilled water), treatment order (order 1, 2 and 3 representing the first, second and last 10 days of the experiment, respectively) or treatment day within each order. Significance levels: p < 0.001 ‘***’, p < 0.01 ‘**’, p < 0.05 ‘*’.
Nest box inspection
Over the experiment, Eastern Rosellas spent a total of 86.5 min inspecting nest boxes by sitting in or near the entrance hole and looking inside. Nest box inspection lasted a mean of 17.48 ± 8.87 s per visit.
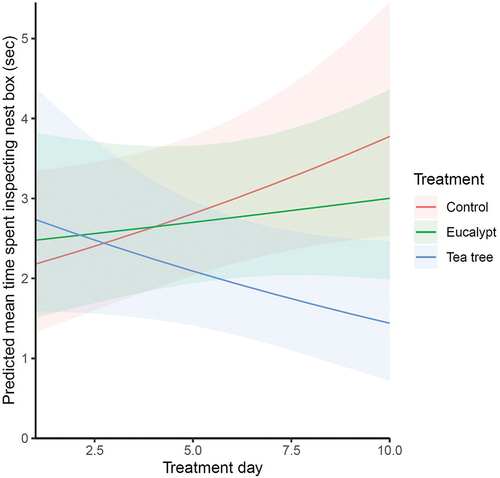
Nest boxes were more likely to receive at least one nest box inspection during visits in the first 10 days (order 1) than the second 10 days (order 2) (estimate = −1.067 ± 0.152, p = 0.030, Supplementary material Table S2, Figure S3). There were no significant results of any factors on the total time spent inspecting each nest box per visit (Supplementary material Table S3). There was no significant effect of eucalyptus scent on the mean time spent inspecting the nest boxes. Eastern Rosellas spent significantly less time inspecting tea tree scented nest boxes as treatment day increased (estimate = −0.091 ± 0.038, p = 0.017, , ).
Figure 4. Effects of plant scent treatment on predicted mean time spent inspecting boxes per visit by Eastern Rosellas depending on treatment day. The mean time spent inspecting boxes slightly increased over the 10-day period following scent placement at boxes with control and eucalypt scents, while it sharply decreased at boxes with tea tree scent. Shaded areas indicate 95% confidence intervals.
Discussion
Although eucalypt and tea tree volatile oils are known to have antiparasitic and antimicrobial benefits (Liu et al. Citation2008; Puvača et al. Citation2019), Eastern Rosellas in this study were not attracted to nest boxes with either scent. This was contradictory to our hypotheses, but also to the findings of Yang et al. (Citation2020) where Russet Sparrows were attracted to the odour of antimicrobial and antiparasitic plant material. In our study, there was no difference in visitation, duration of visits or nest box inspection behaviour between nest boxes scented with eucalypt scent and those scented with the control scent. Eastern Rosellas may have not reacted to the smell of eucalypt because it was familiar to them, as all Australian hollow nesting birds commonly nest in eucalypt trees (Goldingay Citation2009). Blue Mallee was used as the eucalypt scent for this study, although this species is not one of the many Eucalyptus species native to the area, and this could contribute to the lack of reaction from the Eastern Rosellas. However, since many species of Eucalyptus share cineole (1,8-cineole) as a major volatile component in their leaves (Sebei et al. Citation2015), it is unlikely that Eastern Rosellas would have responded differently if the scent of another Eucalyptus species was used. The effect of scent from Eucalyptus species on other birds is an avenue for further study.
Contrary to our hypotheses, Eastern Rosellas were clearly repelled by the scent of tea tree oil in nest boxes: they visited nest boxes less and spent less time (in total and on average) per visit when tea tree scent was present than when it was not present at control boxes. This is the first time birds have been shown to avoid tea tree scents in nest boxes. Potentially, our findings may indicate a neophobic response to an unexpected scent in the nest boxes. Unlike eucalypt leaves which are often added to cavity nests as bedding by Krefft’s Gliders, Squirrel Gliders and Galahs (Eolophus roseicapilla), tea tree is not known as a plant which is regularly found in nesting hollows (Gibbons and Lindenmayer Citation2002). Birds are generally wary of new scents in their environment. For example, unless reared in orange-scented nests, domestic Red Junglefowl chicks (Gallus gallus) avoided food with an orange odour (Turro et al. Citation1994). However, we found that mean inspection time of tea tree scented boxes by Eastern Rosellas was highest immediately following scent addition and decreased in the days after as volatility decreased. This inspection of tea tree scented boxes was in contrast to the behaviour seen at boxes with eucalyptus and the control scents where inspection times increased in the days after scent addition. It seems that Eastern Rosellas were curious about the new tea tree odour in nest boxes but were still repelled by it.
The time after treatment and order of treatment also influenced visitation behaviour of Eastern Rosellas regardless of the scent treatment. Eastern Rosellas generally avoided visiting nest boxes earlier in the treatment days. This may be an effect of human presence when the researcher installed/changed filter papers. Although researchers may not pose a direct threat, study birds will often display fleeing and avoidance behaviours when researchers are present (Götmark Citation1992). Eastern Rosellas also visited nest boxes more frequently in the last 10 days of the experiment compared to the first 10 days of the experiment. Eastern Rosellas may have been increasing visitation frequency to potential nesting sites closer to the beginning of their breeding season in late August. Pell and Tidemann (Citation1997) similarly found that the visit frequency of Eastern Rosellas increased to nest boxes as the breeding season approached. However, Eastern Rosellas spent more time visiting and were more likely to inspect nest boxes in the first 10 days of the experiment compared to the latter 20 days of the experiment. Eastern Rosellas may be reacting to the installation of remote sensing camera and filter paper at the start of the experiment. The effects of camera traps on animal behaviour are relatively unknown, however animals can detect and respond to low-frequency sounds and infrared lights from camera traps (Meek et al. Citation2014). For example, Meek et al. (Citation2016) found that feral cats (Felis catus) displayed listening behaviours as they approached camera traps in arid Australia. The time spent at the nest boxes by Eastern Rosellas may have decreased as they became more familiar with the experimental equipment over time. Future studies should endeavour to instal equipment some time before the experiment starts to reduce behavioural effects of novel objects on study animals.
In Australia, many species of birds and arboreal mammals utilise hollows and nest boxes. As it was unknown which species may visit the newly installed nest boxes, it was beyond the scope of this study to mark birds with unique bands to identify individual birds during the experiment. Therefore, we cannot rule out that the same individuals visited one or more nest boxes multiple times during our scent experiment. However, as we recorded 1454 visits by Eastern Rosellas, it is likely that we studied the behaviours of several individuals and hence we believe that our results are true behavioural responses to the scent treatments. There is also the possibility that individuals were exposed to multiple scents in the same day. Exposure to multiple scents is a fairly common practice in laboratory choice experiments to investigate a preference for one scent over another (see Gwinner and Berger Citation2008; Amo et al. Citation2014, Citation2015). If Eastern Rosellas were exposed to multiple scents in the same day, then the results may indicate stronger individual preferences for particular scents. Future studies on the effects of plant volatiles on birds should be conducted at multiple locations to sample different populations to further understand their responses to scents.
This study was the first to investigate whether Australian birds can smell plant volatiles. It is now widely accepted that olfaction plays an important role in birds’ navigation, food procurement, predator detection and various social behaviours (Abankwah et al. Citation2020). However, the olfactory ability of only a few native Australian species has been investigated. Australian Zebra Finches (Taeniopygia castanotis) use smell to identify individuals of the same species (Krause et al. Citation2014), their kin (Krause et al. Citation2012; Caspers et al. Citation2017) and their own nests (Caspers and Krause Citation2011; Krause et al. Citation2012; Golüke et al. Citation2016). Crimson Rosellas (Platycercus elegans) are able to identify individuals of different subspecies by smell (Mihailova et al. Citation2014). Lastly, female Budgerigars (Melopsittacus undulatus) use smell to distinguish between the odour of male and female Budgerigars (Zhang et al. Citation2010). Our study is a valuable addition to the understanding of olfaction in Australian bird species, but more study is needed to understand how smell can influence bird behaviours.
We found that Eastern Rosellas responded to the smell of tea tree oil and that this scent influenced their behaviour at nest boxes. This finding offers opportunities for wildlife and pest management using scents. Overseas, several studies have shown that scents of predators near nests can repel birds (Amo et al. Citation2008; Stanbury and Briskie Citation2015). However, scent has not been investigated as a tool in bird conservation in Australia. Hollow and nest box competition from both exotic (Common Starlings and Common Mynas, Acridotheres tristis) and common native birds (such as Rainbow Lorikeets, Trichoglossus moluccanus and Eastern Rosellas) often exclude and prevent threatened cavity nesting birds from using nesting hollows (Goldingay et al. Citation2020). More research is needed to test the effects of a variety of scents on excluding unwanted species without affecting the behaviours of threatened birds and mammals. For example, Stanbury and Briskie (Citation2015) found that, in New Zealand Common Starlings refused to enter nest boxes when rat urine was present, but native New Zealand birds did not recognise the scent as threatening and therefore would enter the nest boxes freely. Scents may be a cost-effective method of deterring invasive birds, such as Common Starlings and Common Mynas, from occupying nest boxes and hollows intended for threatened species. The use of scent could also have a potential application in Tasmania where nest predation by Krefft’s Gliders has contributed to the population decline of Swift Parrots (Heinsohn et al. Citation2015). Potentially a scent that repels Krefft’s Gliders but does not affect the birds could be used to protect the nest hollows of Swift Parrots. More research is needed to understand which scents repel or attract different animal species.
The detection of certain scents through olfaction can influence the behaviour of most animals. Olfaction plays an important role in deterring animals from risky areas (Webster et al. Citation2018) or harmful substances, such as toxins (Sarabian et al. Citation2018), or attracting animals to beneficial resources, such as food or mates (Cunningham et al. Citation2009; Cerveira and Jackson Citation2013). Scent has been successfully used as a tool in wildlife management. For example, anal gland and urine scent are an effective method to attract feral cats to monitoring and control stations in arid Australia (Edwards et al. Citation1997; Hanke and Dickman Citation2013). Furthermore, pheromones were used to attract and find feral goats (Capra hircus) during successful eradication efforts on the Galapagos islands (Robertson et al. Citation2017). More recently, odours are increasingly used as a method of insect pest management to reduce the use of agricultural pesticides, both by using plant volatiles to repel insects from crops and using pheromones to attract pest insects to traps (Witzgall et al. Citation2010; Devrnja et al. Citation2022). Our study suggests that scent may be an effective tool in Australian wildlife management, however more research is needed to ensure it is safe and effective.
Supplemental Material
Download MS Word (1 MB)Acknowledgments
We thank the Burrendong Botanic Garden and Arboretum team, and in particular Mike Herbert, for their support and assistance. Deanna Duffy and Simon McDonald from SPAN at Charles Sturt University provided assistance with ArcGIS and R programming. Dale Nimmo for providing extra camera traps.
Disclosure statement
This research has been conducted using Habitech modular nest boxes which are manufactured by Habitech Pty Ltd and solely distributed by Habitat Innovation and Management. Neither business has sponsored, contributed to, or otherwise influenced this research in any manner. However, MC discloses a perceived conflict of interest as a director and shareholder in both companies.
Data availability statement
Data from this study are available at https://doi.org/10.6084/m9.figshare.23930481.v1.
Supplementary material
Supplemental data for this article can be accessed at https://doi.org/10.1080/01584197.2024.2323920.
Additional information
Funding
References
- Abankwah, V., Deeming, D. C., and Pike, T. W. (2020). Avian olfaction: A review of the recent literature. Comparative Cognition & Behavior Reviews 15, 149–161. doi:10.3819/CCBR.2020.150005
- Amo, L., Galván, I., Tomás, G., and Sanz, J. J. (2008). Predator odour recognition and avoidance in a songbird. Functional Ecology 22(2), 289–293. doi:10.1111/j.1365-2435.2007.01361.x
- Amo, L., López-Rull, I., Pagán, I., and García, C. M. (2015). Evidence that the house finch (Carpodacus mexicanus) uses scent to avoid omnivore mammals. Revista Chilena de Historia Natural 88(1), 5. doi:10.1186/s40693-015-0036-4
- Amo, L., Tomás, G., Parejo, D., Avilés, J. M., and Ravel, N. (2014). Are female starlings able to recognize the scent of their offspring? PLoS ONE 9(10), e109505. doi:10.1371/journal.pone.0109505
- Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1–48. doi:10.18637/jss.v067.i01
- Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., and White, J. S. S. (2009). Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution 24, 127–135. doi:10.1016/j.tree.2008.10.008
- Boulton, A. J., and Williford, A. (2018). Analyzing skewed continuous outcomes with many zeros: A tutorial for social work and youth prevention science researchers. Journal of the Society for Social Work and Research 9(4), 721–740. doi:10.1086/701235
- Brooks, M., Kristensen, K., van Benthem, K., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9(2), 378–400. doi:10.32614/RJ-2017-066
- Callan, M. N., Krix, D., McLean, C. M., Murray, B. R., and Webb, J. K. (2023). Thermal profiles of chainsaw hollows and natural hollows during extreme heat events. Biology 12(3), 361. doi:10.3390/biology12030361
- Caspers, B. A., Hagelin, J. C., Paul, M., Bock, S., Willeke, S., and Krause, E. T. (2017). Zebra finch chicks recognise parental scent, and retain chemosensory knowledge of their genetic mother, even after egg cross-fostering. Scientific Reports 7(1), 12859. doi:10.1038/s41598-017-13110-y
- Caspers, B. A., and Krause, E. T. (2011). Odour-based natal nest recognition in the zebra finch (Taeniopygia guttata), a colony-breeding songbird. Biology Letters 7(2), 184–186. doi:10.1098/rsbl.2010.0775
- Cerveira, A. M., and Jackson, R. R. (2013). Love is in the air: Olfaction-based mate-odour identification by jumping spiders from the genus Cyrba. Journal of Ethology 31(1), 29–34. doi:10.1007/s10164-012-0345-x
- Chapman, A. D. (2009). ‘Numbers of living species in Australia and the world.’ Department of the Environment, Water, Heritage and the Arts. Available at https://www.dcceew.gov.au/science-research/abrs/publications/other/numbers-living-species
- Clark, L., and Mason, J. R. (1985). Use of nest material as insecticidal and anti-pathogenic agents by the European starling. Oecologia 67(2), 169–176. doi:10.1007/BF00384280
- Clark, L., and Mason, J. R. (1987). Olfactory discrimination of plant volatiles by the European starling. Animal Behaviour 35(1), 227–235. doi:10.1016/S0003-3472(87)80228-2
- Clark, L., and Mason, J. R. (1988). Effect of biologically active plants used as nest material and the derived benefit to starling nestlings. Oecologia 77, 174–180. doi:10.1007/BF00379183
- Clark, L., and Smeraski, C. A. (1990). Seasonal shifts in odor acuity by starlings. Journal of Experimental Zoology 255(1), 22–29. doi:10.1002/jez.1402550105
- Cunningham, S. J., Castro, I., and Potter, M. A. (2009). The relative importance of olfaction and remote touch in prey detection by North Island brown kiwis. Animal Behaviour 78(4), 899–905. doi:10.1016/j.anbehav.2009.07.015
- De Groof, G., Gwinner, H., Steiger, S., Kempenaers, B., Van der Linden, A., and Hendricks, M. (2010). Neural correlates of behavioural olfactory sensitivity changes seasonally in European starlings. PLoS ONE 5(12), e14337. doi:10.1371/journal.pone.0014337
- Devrnja, N., Milutinović, M., and Savić, J. (2022). When scent becomes a weapon - plant essential oils as potent bioinsecticides. Sustainability 14, 6847. doi:10.3390/su14116847
- Dubiec, A., Góźdź, I., and Mazgajski, T. D. (2013). Green plant material in avian nests. Avian Biology Research 6(2), 133–146. doi:10.3184/175815513X13615363233558
- Edwards, G. P., Piddington, K. C., and Paltridge, R. M. (1997). Field evaluation of olfactory lures for feral cats (Felis catus) in central Australia. Wildlife Research 24, 173–183. doi:10.1071/WR96013
- Gibbons, P., and Lindenmayer, D. B. (2002). ‘Tree Hollows and Wildlife Conservation in Australia.’ (CSIRO: Collingwood, Victoria.)
- Goldingay, R. L. (2009). Characteristics of tree hollows used by Australian birds and bats. Wildlife Research 36(5), 394–409. doi:10.1071/WR08172
- Goldingay, R. L., Rohweder, D., and Taylor, B. D. (2020). Nest box contentions: Are nest boxes used by the species they target? Ecological Management & Restoration 21(2), 115–122. doi:10.1111/emr.12408
- Golüke, S., Dörrenberg, S., Krause, E. T., Caspers, B. A., and Moskát, C. (2016). Female zebra finches smell their eggs. PLoS ONE 11(5), e0155513. doi:10.1371/journal.pone.0155513
- Götmark, F. (1992). The effects of investigator disturbance on nesting birds. In ‘Current Ornithology.’ (Ed. D. M. Power.) pp. 63–104. (Springer: USA.)
- Gwinner, H. (1997). The function of green plants in nests of European starlings (Sturnus vulgaris). Behaviour 134(5–6), 337–351. doi:10.1163/156853997X00575
- Gwinner, H., and Berger, S. (2008). Starling males select green nest material by olfaction using experience-independent and experience-dependent cues. Animal Behaviour 75(3), 971–976. doi:10.1016/j.anbehav.2007.08.008
- Gwinner, H., Capilla-Lasheras, P., Cooper, C., and Helm, B. (2018). ‘Green incubation’: Avian offspring benefit from aromatic nest herbs through improved parental incubation behaviour. Proceedings Biological Sciences 285, 20180376. doi:10.1098/rspb.2018.0376.
- Gwinner, H., Oltrogge, M., Trost, L., and Nienaber, U. (2000). Green plants in starling nests: Effects on nestlings. Animal Behaviour 59(2), 301–309. doi:10.1006/anbe.1999.1306
- Habitat Innovation and Management (n.d.). ‘Habitech nest boxes.’ Available at https://www.habitatinnovation.com.au/habitech-nest-boxes [Verified 12 May 2023].
- Hanke, P. U., and Dickman, C. R. (2013). Sniffing out the stakes: Hair-snares for wild cats in arid environments. Wildlife Research 40(1), 45–51. doi:10.1071/WR12210
- Hansell, M. (2000). ‘Bird Nests and Construction Behaviour.’ (Cambridge University press: Cambridge, UK.)
- Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E. D., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6, e4794. doi:10.7717/peerj.4794
- Hartig, F. (2017). ‘Package ‘DHARMa’.’ Available at https://florianhartig.github.io/DHARMa/ [Verified 20 January 2023].
- Heinsohn, R., Webb, M., Lacy, R., Terauds, A., Alderman, R., and Stojanovic, D. (2015). A severe predator-induced population decline predicted for endangered, migratory swift parrots (Lathamus discolor). Biological Conservation 186, 75–82. doi:10.1016/j.biocon.2015.03.006.
- Hunter, J. T. (2015). Seasonality of climate drives the number of tree hollows in eastern Australia: Implications of a changing climate. International Journal of Ecology 2015, 190637. doi:10.1155/2015/190637
- Kim, S.-I., Yi, J.-H., Tak, J.-H., and Ahn, Y.-J. (2004). Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari: Dermanyssidae). Veterinary Parasitology 120, 297–304. doi:10.1016/j.vetpar.2003.12.016.
- Krause, E. T., Brummel, C., Kohlwey, S., Baier, M. C., Müller, C., Bonadonna, F., and Caspers, B. A. (2014). Differences in olfactory species recognition in the females of two Australian songbird species. Behavioral Ecology and Sociobiology 68(11), 1819–1827. doi:10.1007/s00265-014-1791-y
- Krause, E. T., Caspers, B. A., and Zeil, J. (2012). Are olfactory cues involved in nest recognition in two social species of estrildid finches? PLoS ONE 7(5), e36615. doi:10.1371/journal.pone.0036615
- Krause, E. T., Krüger, O., Kohlmeier, P., and Caspers, B. A. (2012). Olfactory kin recognition in a songbird. Biology Letters 8(3), 327–329. doi:10.1098/rsbl.2011.1093
- Lambrechts, M. M., and Dos Santos, A. (2000). Aromatic herbs in Corsican blue tit nests: The ‘potpourri’ hypothesis. Acta Oecologica 21(3), 175–178. doi:10.1016/S1146-609X(00)00122-3
- Lindenmayer, D. B., Laurance, W. F., Franklin, J. F., Likens, G. E., Banks, S. C., Blanchard, W., et al. (2014). New policies for old trees: averting a global crisis in a keystone ecological structure. Conservation Letters 7(1), 61–69. doi:10.1111/conl.12013
- Liu, X., Chen, Q., Wang, Z., Xie, L., and Xu, Z. (2008). Allelopathic effects of essential oil from Eucalyptus grandis × E. urophylla on pathogenic fungi and pest insects. Frontiers of Forestry in China 3(2), 232–236. doi:10.1007/s11461-008-0036-5
- Lüdecke, D., Aust, F., Crawley, S., and Ben-Shachar, M. (2020). ‘Package ‘ggeffects’.’ Available at https://cran.r-project.org/web/packages/ggeffects/index.html [Verified 20 January 2023].
- Manning, A. D., Gibbons, P., Fischer, J., Oliver, D. L., and Lindenmayer, D. B. (2013). Hollow futures? Tree decline, lag effects and hollow-dependent species. Animal Conservation 16(4), 395–403. doi:10.1111/acv.12006
- McGuire, A., and Kleindorfer, S. (2007). Nesting success and apparent nest-adornment in diamond firetails (Stagonopleura guttata). Emu - Austral Ornithology 107(1), 44–51. doi:10.1071/MU06031
- Meek, P., Ballard, G., Fleming, P., and Falzon, G. (2016). Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecology and Evolution 6(10), 3216–3225. doi:10.1002/ece3.2111
- Meek, P. D., Ballard, G.-A., Fleming, P. J. S., Schaefer, M., Williams, W., and Falzon, G. (2014). Camera traps can be heard and seen by animals. PLoS ONE 9, e110832. doi:10.1371/journal.pone.0110832
- Mennerat, A., Perret, P., Bourgault, P., Blondel, J., Gimenez, O., Thomas, D. W., et al. (2009). Aromatic plants in nests of blue tits: Positive effects on nestlings. Animal Behaviour 77(3), 569–574. doi:10.1016/j.anbehav.2008.11.008
- Mennerat, A. L., Perret, P., Caro, S. P., Heeb, P., and Lambrechts, M. M. (2008). Aromatic plants in blue tit cyanistes caeruleus nests: No negative effect on blood-sucking protocalliphora blow fly larvae. Journal of Avian Biology 39, 127–132. doi:10.1111/j.0908-8857.2008.04400.x
- Merino, S., and Potti, J. (1995). Mites and blowflies decrease growth and survival in nestling pied flycatchers. Oikos 73(1), 95–103. doi:10.2307/3545730
- Metcalf, E. C., Ross, T., and Metcalf, R. (1989). Nest structure of the collared sparrowhawk “Accipiter cirrocephalus”. Australian Bird Watcher 13, 32–34. doi:10.3316/informit.594205472050450.
- Mihailova, M., Berg, M. L., Buchanan, K. L., and Bennett, A. T. D. (2014). Odour-based discrimination of subspecies, species and sexes in an avian species complex, the crimson rosella. Animal Behaviour 95, 155–164. doi:10.1016/j.anbehav.2014.07.012
- Møller, A. P., Flensted-Jensen, E., Mardal, W., and Soler, J. J. (2013). Host-parasite relationship between colonial terns and bacteria is modified by a mutualism with a plant with antibacterial defenses. Oecologia 173(1), 169–178. doi:10.1007/s00442-013-2600-4
- Newkirk, E. S. (2016). ‘CPW photo warehouse.’ Available at http://cpw.state.co.us/learn/Pages/ResearchMammalsSoftware.aspx [Verified 10 June 2022].
- Pell, A. S., and Tidemann, C. R. (1997). The impact of two exotic hollow-nesting birds on two native parrots in savannah and woodland in eastern Australia. Biological Conservation 79(2–3), 145–153. doi:10.1016/S0006-3207(96)00112-7
- Petit, C., Hossaert-McKey, M., Perret, P., Blondel, J., and Lambrechts, M. M. (2002). Blue tits use selected plants and olfaction to maintain an aromatic environment for nestlings. Ecology Letters 5(4), 585–589. doi:10.1046/j.1461-0248.2002.00361.x
- Pires, B. A., Belo, A. D. F., Diamantino, F., Rabaça, J. E., and Merino, S. (2020). Development of nestling blue tits (Cyanistes caeruleus) is affected by experimental addition of aromatic plants. Avian Biology Research 13(3), 44–48. doi:10.1177/1758155920921075
- Potvin, D. A., Opitz, F., Townsend, K. A., and Knutie, S. A. (2021). Use of anthropogenic-related nest material and nest parasite prevalence have increased over the past two centuries in Australian birds. Oecologia 196(4), 1207–1217. doi:10.1007/s00442-021-04982-z
- Puvača, N., Čabarkapa, I., Petrović, A., Bursić, V., Prodanović, R., Soleša, D., and Lević, J. (2019). Tea tree (Melaleuca alternifolia) and its essential oil: antimicrobial, antioxidant and acaricidal effects in poultry production. World’s Poultry Science Journal 75, 235–246. doi:10.1017/S0043933919000229.
- R Core Team. (2022). R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing: Vienna, Austria.)
- Robertson, B. A., Ostfeld, R. S., and Keesing, F. (2017). Trojan females and Judas goats: Evolutionary traps as tools in wildlife management. BioScience 67(11), 983–994. doi:10.1093/biosci/bix116
- Sarabian, C., Belais, R., and MacIntosh, A. J. J. (2018). Feeding decisions under contamination risk in bonobos. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 20170195. doi:10.1098/rstb.2017.0195
- Scott-Baumann, J. F., and Morgan, E. R. (2015). A review of the nest protection hypothesis: Does inclusion of fresh green plant material in birds’ nests reduce parasite infestation? Parasitology 142(8), 1016–1023. doi:10.1017/S0031182015000189
- Sebei, K., Sakouhi, F., Herchi, W., Khouja, M. L., and Boukhchina, S. (2015). Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biological Research 48(1), 7. doi:10.1186/0717-6287-48-7
- Stanbury, M., and Briskie, J. V. (2015). I smell a rat: Can New Zealand birds recognize the odor of an invasive mammalian predator? Current Zoology 61(1), 34–41. doi:10.1093/czoolo/61.1.34
- Symonds, M. R. E., and Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology 65, 13–21. doi:10.1007/s00265-010-1037-6
- Tomás, G., Merino, S., Martínez-de la Puente, J., Moreno, J., Morales, J., Lobato, E., et al. (2012). Interacting effects of aromatic plants and female age on nest-dwelling ectoparasites and blood-sucking flies in avian nests. Behavioural Processes 90(2), 246–253. doi:10.1016/j.beproc.2012.02.003
- Turro, I., Porter, R. H., and Picard, M. (1994). Olfactory cues mediate food selection by young chicks. Physiology & Behavior 55(4), 761–767. doi:10.1016/0031-9384(94)90057-4
- Veiga, J. P., Polo, V., and Viñuela, J. (2006). Nest green plants as a male status signal and courtship display in the spotless starling. Ethology 112(2), 196–204. doi:10.1111/j.1439-0310.2006.01148.x
- Webster, C., Massaro, M., Michael, D. R., Bambrick, D., Riley, J. L., and Nimmo, D. G. (2018). Native reptiles alter their foraging in the presence of the olfactory cues of invasive mammalian predators. Royal Society Open Science 5(10), 180136. doi:10.1098/rsos.180136
- Witzgall, P., Kirsch, P., and Cork, A. (2010). Sex pheromones and their impact on pest management. Journal of Chemical Ecology 36(1), 80–100. doi:10.1007/s10886-009-9737-y
- Yang, C., Ye, P., Huo, J., Møller, A. P., Liang, W., and Feeney, W. E. (2020). Sparrows use a medicinal herb to defend against parasites and increase offspring condition. Current Biology 30(23), R1411–R1412. doi:10.1016/j.cub.2020.10.021
- Zabłotni, A., Kaliński, A., Bańbura, M., Glądalski, M., Markowski, M., Skwarska, J., et al. (2020). Experimental nest replacement suggests that the bacterial load of nests may mediate nestling physiological condition in cavity nesting great tits (Parus major). Journal of Ornithology 161(3), 819–828. doi:10.1007/s10336-020-01759-8
- Zhang, J.-X., Wei, W., Zhang, J.-H., and Yang, W.-H. (2010). Uropygial gland-secreted alkanols contribute to olfactory sex signals in budgerigars. Chemical Senses 35(5), 375–382. doi:10.1093/chemse/bjq025

