ABSTRACT
Body condition in individuals is intricately linked to behaviour, physiology, and immunity. This study investigated how seasonal factors, such as reproduction and moulting, influence the body condition of 21 tanager species in the Atlantic Forest. Utilising publicly available data, I employed phylogenetic generalised linear mixed models to dissect these influences. Notably, females with brood patches exhibited concurrent body and feather moult, suggesting simultaneous energy-costly constraints. Furthermore, females with brood patches displayed higher body condition, indicating proactive preparation or increased resource availability during breeding. Lastly, individuals moulting body feathers exhibited enhanced body condition, suggesting heightened foraging activity. These data suggest complex connections between sex, moulting, and reproductive status in tanagers in the Atlantic Forest.
Introduction
Energy storage is a vital facet of the annual cycle of birds, with heightened significance during periods of elevated energy expenditure, for example during migration, reproduction, starvation, and infection (Houston and McNamara Citation1993; Gentle and Gosler Citation2001; Araújo et al. Citation2019; Jiménez-Peñuela et al. Citation2019; Ruhs et al. Citation2020). Consequently, body condition emerges as a valuable proxy for gauging the overall well-being of individuals (Peig and Green Citation2010). For example, in species like Ruddy Ground Doves (Columbina talpacoti), Great Kiskadees (Pitangus sulphuratus), Lesser Elaenias (Elaenia chiriquensis), Flavescent Warblers (Myiothlypis flaveola), and Brown-Crested Flycatchers (Myiarchus tyrannulus), individuals with lower body condition exhibit heightened susceptibility to haemosporidian parasites (da Silva Rodrigues et al. Citation2021).
The relationship between body condition and survival can vary with age and sex. Juveniles typically manifest lower body conditions relative to adults (Jones et al. Citation2002). Furthermore, either males or females or neither sex can show higher body condition: (a) males exhibit superior body condition relative to females in Red-Legged Partridges (Alectoris rufa) (Nadal et al. Citation2018), whereas (b) females display elevated body condition in comparison to males in Willow Flycatchers (Empidonax traillii) (Owen et al. Citation2005). In contrast, there can also be a lack of conspicuous differences between sexes (Scherer et al. Citation2014; Angel et al. Citation2015; Wojczulanis-Jakubas et al. Citation2015), or a complicated interaction between sex and age, exemplified in Swallow-Tailed Manakins (Chiroxiphia caudata), in which juvenile males surpass females in body condition, while adult females outperform adult males (Souza Penha and da Silva Rodrigues Citation2022).
These observed variations underscore the potential influence of ancillary factors, notably regional differences between temperate and tropical regions. Tropical bird species potentially face heightened threats, including a higher risk of extinction (Reif and Štěpánková Citation2016) and lower heat tolerance (Pollock et al. Citation2021). Consequently, they are important species for investigating body condition dynamics, particularly in the context of climate change scenarios (Şekercioğlu et al. Citation2012).
Feather moult constitutes a systematic and seasonally recurring process in avian life, representing a crucial aspect for birds of diverse age groups (Gill Citation2007). This process is energetically demanding and is closely linked to physiological trade-offs, where factors such as migration and reproduction can impose constraints on feather moult (Murphy Citation1996). Given its substantial energy expenditure, moult holds the potential to impact the overall body condition of birds. In temperate bird species, empirical evidence supports this notion, for example in Barnacle Geese (Branta leucopsis) (Portugal et al. Citation2007) and Captive Starlings (Sturnus vulgaris) (Swaddle and Witter Citation1997). However, it is important to determine whether similar relationships are manifest in tropical avian species. For example, previous studies have found a negative link between stress, measured through plasma corticosterone, and body condition in tropical populations of Long-Tailed Finches during the moult period (Poephila acuticauda, and P. personata) (Maute et al. Citation2013).
In tandem with the moulting season, reproduction represents another energetically demanding phase in the avian life cycle. Some species strategically moult their feathers before the breeding season (Wolfe et al. Citation2009, Citation2010, Citation2012). Subsequently, the breeding season itself involves a suite of resource-intensive behaviours, including decisions on breeding location, nest construction, surveillance of nest and nestlings, and foraging activities (Smith et al. Citation2007; Slagsvold and Wiebe Citation2011; Mainwaring and Hartley Citation2013; Morinay et al. Citation2018; Williams Citation2018; Martínez et al. Citation2022). A study encompassing 20 bird species in Finland found that females tended to exhibit a lower body condition towards the conclusion of the breeding season, suggesting a potential constraint on female body condition imposed by reproductive efforts (Andersson et al. Citation2018). However, moult and breeding periods may also overlap, as demonstrated in tropical Amazonian bird furnariids and thamnophilids (Johnson et al. Citation2012), suggesting that there might even be a synergistic detrimental effect of breeding and moulting on the body condition of birds.
In this study, I examine the impact of sex, feather moult (body and flight feathers), and brood patch presence on body condition in 21 different tanagers (Passeriformes: Thraupidae) in the Atlantic Forest Biome. While previous research in Amazonian tanagers suggests a lack of overlap between moult and breeding (Johnson et al. Citation2012), I explored the potential synergistic effects of simultaneous moulting and breeding on tanager body condition. This study utilised a comprehensive database of tanager species in the Atlantic Forest Biome, captured across various Brazilian states and years (Rodrigues et al. Citation2019). Predictions included a negative effect of moult on body condition due to the energy trade-off between feather replacement and fat reserve deposition. Additionally, individuals with a brood patch were expected to exhibit a higher body condition to cope with the energy trade-offs associated with reproduction.
Methods
Dataset
This study utilised data from the Atlantic bird traits dataset, a compilation of data from 711 bird species across South America (Rodrigues et al. Citation2019). The dataset encompasses spatial, temporal, and morphometric characteristics, including various length measurements, body mass, and capture details for each individual. My focus here is on 21 tanager species, namely (total/used sample sizes in parentheses): Coryphospingus cucullatus (148/39), Cyanerpes cyaneus (83/35), Dacnis cayana (377/37), Haplospiza unicolor (958/305), Poospiza cabanisi (299/31), Ramphocelus bresilius (204/39), Sicalis flaveola (340/90), Sporophila caerulescens (196/38), Stephanophorus diadematus (95/16), Tachyphonus coronatus (1033/302), Tachyphonus cristatus (104/23), Tachyphonus rufus (1087/518), Tangara cayana (894/234), Tangara cyanocephala (84/22), Tangara peruviana (52/18), Tangara seledon (101/28), Thraupis bonariensis (30/12), Thraupis ornata (43/10), Tiaris fuliginosus (299/14), Trichothraupis melanops (1493/304), and Volatinia jacarina (191/18), totalling 2133 individuals. To ensure data quality, I adhered to the scientific names outlined by Jetz et al. (Citation2012), and excluded data records lacking data on essential variables: body mass, total tail length, sex, age, presence of flight feather moult, presence of body feathers moult, capture date, presence of brood patch, and Brazilian state. Data were also excluded from individuals from states and years with a sample size below 10, as well as juveniles due to limited sample size in certain Brazilian states (Supplementary Tables S1, 2, and 3 – more information in the Body condition section).
Age determination was based on plumage patterns, commissure presence, iris colouration, or ossification level, while sex determination considered age and plumage (Rodrigues et al. Citation2019). Flight feather moult was identified by actively growing symmetrical or asymmetrical flight feathers, and body feather moult was recognised by growing feathers in any body area except the wings. I included both flight and body feather moults as separate variables because of their differential functional significance. Finally, a breeding season variable was established based on capture date. Captures from October to March were considered to be in the breeding season, and those from April to September as non-breeding, aligning with neotropical passerine patterns (IBAMA Citation1994; Gill Citation2007).
Finally, I conducted a thorough investigation of the overlap between the presence of brood patch, body moult, and flight feather moult, separated by sex. Therefore, I found 238 males that were labelled as having a brood patch (reproductive_state variable in the dataset). The dataset also provides specifically the stage in which the brood patch was found (brood_patch variable in the dataset). I found that 225 individuals did not have information on the brood patch stage, 12 individuals had a stage 0, meaning that the brood patch was absent, and one individual had a brood patch stage as 5. Brood patch stage followed the regulations for bird banding procedures from the Centro Nacional de Pesquisa e Conservaҫão de Aves Silvestres (CEMAVE), which stablished 6 stages: 0 absence of brood patch; 1: few lost feathers and low level of vascularisation; 2: clear vascularisation; 3: extreme vascularisation with a thick skin; 4: loss of vascularisation but still with a thick skin; 5: absence of vascularisation and the presence of growing feather sin the region (Souza and Serafini Citation2020). The male individual labelled with a brood patch at stage 5 was Tangara cayana, which is mostly known for the female only having a brood patch (Duca and Marini Citation2011). Therefore, due to the uncertainty of these 238 male individuals, I decided to remove them from the statistical analysis. After, I checked the proportion of females that had a brood patch, regardless of the stage, and that were moulting the flight and body feathers. Male individuals removed from statistical analysis can be found in Supplementary Table 5.
Study groups
Tanagers (Passeriformes: Thraupidae) stand as denizens of the Neotropics, spanning from Central to South America, and occur in diverse habitats, including lowland rainforests, grasslands, and high-altitude fields (Rosenberg et al. Citation1999; Rodríguez-Ruíz et al. Citation2011; Eisermann et al. Citation2011a; Rodrigues et al. Citation2019; Winkler et al. Citation2020; Aguiar de Souza Penha et al. Citation2022, Citation2023). Tanagers are believed to be socially monogamous, with both sexes participating in parental care, with clutch size typically ranging from 1–4 eggs and an incubation period lasting 12 to 14 days, but with a high species variation (Aguiar de Souza Penha et al. Citation2022, Citation2023). The incubation is usually performed by the female (Klatt et al. Citation2008; Dos Santos and Marini Citation2010; Duca and Marini Citation2011; Eisermann et al. Citation2011b; Snchez Martínez and Londoño Citation2012; Sánchez-Martínez and Londoño Citation2017; Cerón-Cardona et al. Citation2018; Mortensen and Reed Citation2018; Veloso et al. Citation2018; Rodrigues et al. Citation2019; Loaiza-Muñoz and Londoño Citation2020). We still have little knowledge about the moulting dynamics of tanagers, but studies in the region analysing passerines found that flight feather moult usually takes place at the end or after the breeding season (Marini and Durães Citation2001; Magalhães et al. Citation2007; de Andrade et al. Citation2018; Faccio et al. Citation2018; Rodrigues et al. Citation2019), but with body moult happening throughout the year (Rodrigues et al. Citation2019). While the majority of tanager species are currently categorised as least concerned by the IUCN, the escalating threats of habitat loss and introduction of exotic species have contributed to an increased number of threatened species in recent years (Winkler et al. Citation2020). The phylogeny used in this study follows Jetz et al. (Citation2012).
Body condition
I derived estimates of body condition for all species by analysing residuals from a mass-tail length regression model (Peig and Green Citation2010) implemented through generalised linear mixed models (GLMM). This analysis was conducted using the lmer function from the lme4 package (Bates et al. Citation2015) in the R software (R Core Team Citation2019). In the modelling process, I incorporated breeding season, year of capture, age, and sex as random factors whenever applicable. Also, to eliminate undesired spatial-temporal variability, I computed body condition values for each species and within each Brazilian state, following established methodologies (da Silva Rodrigues et al. Citation2021; Souza Penha and da Silva Rodrigues Citation2022).
Statistical analysis
First, I conducted a thorough investigation of the overlap between the presence of brood patch, body moult, and flight feather moult in females only. Then, to understand the predictors of body condition in tanagers, I conducted a visual examination of the body condition distribution through a histogram and employed the normalize function from the BBmisc package (Bischl et al. Citation2022) to achieve a normalised distribution of the body condition. The multicollinearity among predictors was assessed by calculating the variance inflation factor (VIF) using the VIF function from the regclass package (Petrie Citation2020). A GVIF(1/2df) value of two was considered indicative of multicollinearity. I used a generalised linear mixed model with the lmer function from the lme4 package (Bates et al. Citation2015), with the normalised body condition variable as the response, and the brood patch presence, sex, flight feather moult, and the body feather moult as predictors. Models included the interaction between sex and flight feather moult, body and flight feather moults, and presence of brood patch and body feather moult. Random terms were also included, namely: the longitude, latitude, the breeding status, year, and the species identity. The interaction between flight feather moult and body moult (VIF = 4.05), and between sex and flight feather moult (VIF = 2.61) were highly collinear, so I removed those interactions from the model. After that removal, no collinearity was found among the tested predictors (VIF values: Presence of brood patch = 1.32; Sex = 1.18; Flight moult = 1.18; Body moult = 1.28; Interaction between presence of brood patch and body moult = 1.19). For final modelling, a phylogenetic generalised linear mixed model (PGLMM) was employed utilising the pglmm function from the phyr package (Ives et al. Citation2020), using the final model after verifying for multicollinearity. The response variable was body condition, with the predictors including sex, occurrence of flight moult, occurrence of body moult, presence of brood patch, and the interaction between presence of brood patch and body feather moult. The interaction terms in the model were reported only if statistically significant. To address potential spatial autocorrelation and temporal variability, the same random factors, namely latitude, longitude, breeding season (outside the breeding season and inside the breeding season), and year of capture were incorporated into the model. Additionally, the species phylogeny was utilised to account for species relatedness (Jetz et al. Citation2012). Model comparison involved evaluating the full model against a null model, with the full model deemed statistically relevant only if significantly different from the null model (lower AIC value). To assess model performance, a plot of average residuals versus average fitted values was generated using the binnedplot function from the arm package (Gelman and Su Citation2022) (Supplemental Figure S1). Finally, to consider a variable as statistically significant, I consider the 95% confidence interval as not including zero, and the p-value as being lower than 0.05. To produce the 95% confidence interval, a bootstrap procedure with 1000 iterations was used. For visualisation of statistically significant variables, I used the ggplot2 package with predicted values from the model, using the predict function form R (R Core Team Citation2019), as well as the forestplot function from the forestplot package (Gordon and Lumley Citation2022). Mapping of sampling locations was accomplished using the shape files from IBGE (IBGE - Instituto Brasileiro de Geografia e estatística Citation2013), and the ggplot2 package (Wickham Citation2016). All the analysis were conducted in the R software (R Core Team Citation2019).
Results
Overlap between brood patch and moult
The mean body condition across all species was 0.002 ± 2.32 (mean ± standard error) (). The range in values of condition was dramatic, with one individual of Trichothraupis melanops exhibiting the highest condition in São Paulo state (24.76), while one individual of Tachyphonus rufus had the lowest condition value in Pernambuco (−14.4). Very few females moulted feathers during the breeding season (). Only 3% of females that had a brood patch were also moulting the flight feathers, with Haplospiza unicolor having the highest number of individuals (3 individuals), whereas 12% of females that had a brood patch were also moulting the body feathers, with Sicalis flaveola having the highest number of individuals (6 individuals). In addition, I found 1% of females moulting both flight and body feathers while having a brood patch, being found only in two species (number of individuals in parenthesis), namely Haplospiza unicolor (2), and Sporophila caerulescens (1). A complete summary can be found in Supplementary Table S1. In addition, most females with a brood patch were distributed in the breeding season, from October to March, whereas females without a brood patch were captured through the whole year, with a slightly higher number outside the breeding season, from April to September (Supplementary Table S7).
Figure 1. Map of Brazil and the capture sites of all 2,127 individuals belonging to 21 tanager species (Passeriformes: Thraupidae) incorporated in this study. The black dots on the map denote the sampling locations. Detailed information regarding each location site and the number of captured individuals per species is provided in Supplementary Table 6 for comprehensive reference.
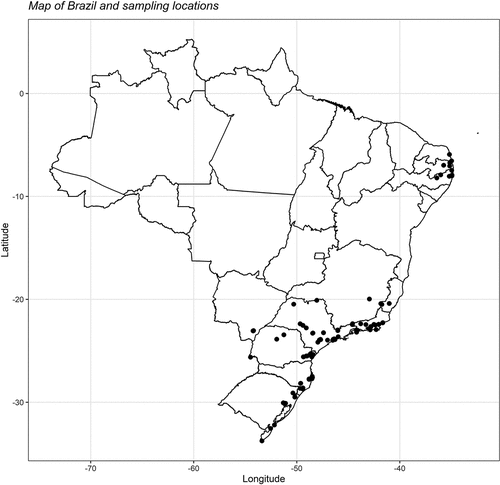
Table 1. Summary data of 953 female individuals belonging to 21 tanager species. Here I show the number of females per species, detailing the total count of captures (N), the number of females undergoing flight feather moult (FM), body moult (BM), or both, categorised by the presence or absence of a brood patch.
Predictors of body condition
The full model (AIC = 5924.66) exhibited a lower AIC value compared to the null model (AIC = 5971.80). The presence of a brood patch and body moult were associated with body condition, such that females that had a brood patch and those individuals moulting the body feathers had a higher body condition compared to females without a brood patch and non-moulting individuals, respectively (, ). There was no significant association of the interaction between brood patch presence and body moult, as well as for sex and flight feather moult ().
Figure 2. Results of the phylogenetic generalized linear mixed model (PGLMM) examining body condition in 2,127 individuals belonging to 21 tanagers species. The PGLMM model had body condition as the response variable, and the presence of a brood patch (reference level: absent), sex (reference level: female), flight feather moult (reference level: absent), and body feather moult (reference level: absent) as predictors. The tanager phylogeny was included in the model to account for species-relatedness. I also included the latitude, longitude, Brazilian state, and year as random terms, to account for potential spatial-temporal variability in the model. Here, I show the 95% confidence intervals (C.I.), with the black box indicating the average C.I., and the straight line indicating the lower (beginning) and upper (end) C.I. Results indicate that females with a brood patch have a higher body condition compared to females without a brood patch and that individuals moulting the body feathers also had a higher body condition compared to non-moulting individuals.
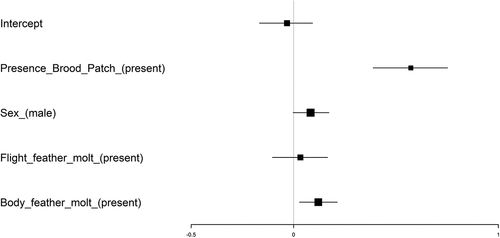
Table 2. Results of the Phylogenetic Generalized Linear Mixed Model (PGLMM) of 2,127 individuals belonging to 21 tanager species with body condition as the response variable, while predictors included sex, presence of body feather moult, flight feather moult, and breeding status. I present fixed-term effects, along with estimates, standard errors (SE), 95% confidence intervals (95% C.I.), and corresponding p-values. Random-effect terms (longitude, latitude, Brazilian, state, breeding season, and year) are delineated by variance and standard deviations. A phylogenetic effect was also taken into consideration to account for species relatedness (phylogenetic effect), showing the variance and standard deviation of the species effect within the model.
Discussion
In this study, there was an observed association of female tanagers undergoing moult (both body and flight feathers) and those exhibiting a brood patch. This observation diverges from a prior study on Amazonian tanagers (Johnson et al. Citation2012), where the analysis focused solely on primary feather moult. Despite methodological differences, that study covered eight tanager species with a substantial sample size (708 individuals), including Cyanocompsa cyanoides, Coereba flaveola, Lanio fulvus, Oryzoborus angolensis, Ramphocelus carbo, Tachyphonus cristatus, Tachyphonus surinamus, and Volatinia jacarina. By narrowing our comparison to species shared between both studies (Tachyphonus cristatus and Volatinia jacarina), our findings align, as in the present study, these species did not show an overlap between flight feather moult and the presence of a brood patch. However, the varying number of females exhibiting concurrent brood patch and moulting – whether flight feather, body feather, or both – suggests potential differences among tanager populations.
I observed that individuals displaying a brood patch exhibited a higher body condition compared to those without. This outcome suggests that females with a brood patch during the breeding season may enjoy enhanced resource availability. It is worth noting that the breeding season may coincide with the wet season and peaks of flowering, leaf flushes, and resource abundance in the Atlantic Forest (Develey and Peres Citation2000; Morellato et al. Citation2000). As an example of other tropical groups, in years marked by increased rainfall and potentially higher resource availability, female White-Rumped Munias (Lonchura striata), experienced heightened overall body conditions, leading to consequential increases in clutch size and female fecundity (Oppel et al. Citation2013; Hidalgo Aranzamendi et al. Citation2019). Females may strategically enhance their body condition before and during the breeding season, possibly to better cope with the various stressors associated with reproduction. For instance, in temperate species, females with stage 2 brood patches exhibited superior body conditions during the breeding season (Redfern Citation2010). Similarly, in Common Bulbuls (Pycnonotus barbatus), a tropical species, individuals experienced a peak in body mass during the incubation period, even though it was not specifically correlated with brood patch development (Nwaogu et al. Citation2017). Female tanagers in Atlantic Forest may derive benefits from synchronising the breeding season with the peak of resource availability, as reflected in their elevated body condition.
I observed that individuals undergoing body feather moult exhibited a higher body condition compared to those not moulting, a finding contrary to my initial expectations. However, a parallel trend was found in a study of House Finches (Haemorhous mexicanus) inhabiting the Arizona desert, USA. In that study, male House Finches with elevated body conditions displayed more intense body feather moulting compared to their urban counterparts (Hutton et al. Citation2021). A similar association was noted among migratory Barn Swallows (Hirundo rustica) wintering in Nigeria (Saino et al. Citation2013). These results suggest two non-mutually exclusive hypotheses. First, individuals undergoing body feather moult may actively seek energy-rich resources. Second, it’s plausible that individuals attain a higher body condition before the onset of moulting to better cope with the energy expenditures associated with replacing body feathers.
Finally, no discernible associations between sex and body condition emerged in my study. In earlier studies, findings varied between males exhibiting higher body condition than females (Nadal et al. Citation2018), females demonstrating higher body condition than males (Owen et al. Citation2005), and even the potential influence of age influencing the dynamics between body condition and sex (Souza Penha and da Silva Rodrigues Citation2022). This suggests that in tanagers, both males and females might share similar behavioural constraints linked to securing energy-rich resources or encountering physiological trade-offs associated with reproduction, migration, and moulting. Interestingly, individuals undergoing flight feather moult displayed a comparable body condition to non-moulting counterparts, hinting that the moulting process might not exert substantial costs. However, recent studies have proposed vital connections between moult, plasma proteins, and circulating triglyceride levels – significant indicators of nutrient status in individuals (Podlaszczuk et al. Citation2017; Drake and McGraw Citation2023). To enhance our understanding of the energy dynamics during moulting periods in tanagers, I recommend that future studies measure critical circulating determinants of body condition.
It’s essential to acknowledge some limitation in my findings – the inability to distinguish between asymmetrical and symmetrical flight feather moults. Asymmetrical moults are typically associated with feather loss, while symmetrical moults are linked to the seasonal process of feather replacement (Gill Citation2007; Rodrigues et al. Citation2019). Furthermore, given the limited sample size of juveniles in certain Brazilian states, I was unable to explore the relationship between age and sex in relation to body condition. Therefore, future research endeavours should prioritise investigating how feather moult impacts body condition while considering various age groups, particularly juvenile birds.
In summary, this study revealed a noteworthy convergence between female tanagers undergoing moult and those exhibiting a brood patch, suggesting potential strategic advantages tied to the breeding season. Additionally, individuals with a brood patch displayed elevated body condition, indicating an adaptive response linked to resource availability during the Atlantic Forest’s wet season. This pattern aligns with strategic behaviours observed in diverse temperate and tropical bird species during their respective breeding periods, underscoring the significance of timing and resource optimisation. Unexpectedly, individuals undergoing body feather moult exhibited higher body condition, challenging our initial expectations and implying resource-seeking behaviours or proactive readiness for the energy-intensive feather replacement process. To extend these findings, future research could delve into tanager dietary patterns, energy allocation during different moult and breeding stages, and the timing and duration of moult events to gain deeper insights into their ecological implications.
Supplemental Material
Download MS Word (238.3 KB)Acknowledgments
VAP expresses gratitude to the Organismal and Evolutionary Research Programme of the University of Helsinki, as well as the European Union fellowship that provided funding for the Identification of Best Practices for Biodiversity Recovery and Public Health Interventions to Prevent Future Epidemics and Pandemics (BEPREP) project, including support for VAP during the development of this study. I acknowledge the important contributions and thorough reviews provided by the Editor and Reviewers associated with Emu–Austral Ornithology. Their insights and suggestions have significantly enhanced the comprehensiveness of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The dataset is available in the literature (Rodrigues et al. Citation2019), while the corresponding R code can be accessed from the GitHub repository at https://github.com/victoraspenha/body-condition-in-tanagers.
Supplementary material
Supplemental data for this article can be accessed at https://doi.org/10.1080/01584197.2024.2328675.
References
- Aguiar de Souza Penha, V., Maia Chaves Bicalho Domingos, F., Fecchio, A., Bell, J. A., Weckstein, J. D., Ricklefs, R. E., et al. (2022). Haemosporidian parasites and incubation period influence plumage coloration in tanagers (Passeriformes: Thraupidae). Proceedings of the Royal Society B: Biological Sciences 289. doi:10.1098/rspb.2022.1283
- Aguiar de Souza Penha, V., Maia Chaves Bicalho Domingos, F., Fecchio, A., Bell, J. A., Weckstein, J. D., Ricklefs, R. E., et al. (2023). Host life-history traits predict haemosporidian parasite prevalence in tanagers (Aves: Thraupidae). Parasitology 150, 32–41. doi:10.1017/S0031182022001469
- Andersson, N., Piha, M., Meller, K., Välimäki, K., and Lehikoinen, A. (2018). Variation in body condition of songbirds during breeding season in relation to sex, migration strategy and weather. Ornis Fennica 95(2), 70–81. doi:10.51812/of.133931
- Angel, L. P., Wells, M. R., Rodríguez-Malagón, M. A., Tew, E., Speakman, J. R., Arnould, J. P. Y., and Ambrosini, R. (2015). Sexual size dimorphism and body condition in the Australasian gannet. PLoS One 10(12), 1–16. doi:10.1371/journal.pone.0142653
- Araújo, P. M., Viegas, I., Rocha, A. D., Villegas, A., Jones, J. G., Mendonça, L., et al. (2019). Understanding how birds rebuild fat stores during migration: Insights from an experimental study. Scientific Reports 9(1), 10065. doi:10.1038/s41598-019-46487-z
- Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1–48. doi:10.18637/jss.v067.i01
- Bischl, B., Lang, M., Bossek, J., Horn, D., Richter, J., and Surmann, D. (2022). ‘BBmisc: Miscellaneous helper functions for B. Bischl. R package version 1.13.’ Available at https://CRAN.R-project.org/package=BBmisc
- Cerón-Cardona, J., Vooz, J. P., and Londoño, G. A. (2018). Nesting biology of the White-winged shrike-tanager (Lanio versicolor). The Wilson Journal of Ornithology 130(3), 639–649. doi:10.1676/17-050.1
- da Silva Rodrigues, R., de Souza Penha, V. A., Miwa, R. Y., Branco, J. O., and Junior, O. M. (2021). Stress and body condition predict haemosporidian parasitaemia in birds from Cerrado, southeastern Brazil. Ardea 109. doi:10.5253/arde.v109i3.a7
- de Andrade, P. G. B., Moreno, D. J., Melo, M. A., Ribeiro, B. C., and Piratelli, A. J. (2018). Bird molting and breeding in an area undergoing re-vegetation in the Atlantic Forest of southeastern Brazil. Revista Brasileira de Ornitologia 26, 141–148. doi:10.1007/BF03544424
- Develey, P. F., and Peres, C. A. (2000). Resource seasonality and the structure of mixed species bird flocks in a coastal Atlantic Forest of southeastern Brazil. Journal of Tropical Ecology 16(1), 33–53. doi:10.1017/S0266467400001255
- Dos Santos, L. R., and Marini, MÂ. (2010). Breeding biology of White-rumped Tanagers in central Brazil. Journal of Field Ornithology 81(3), 252–258. doi:10.1111/j.1557-9263.2010.00280.x
- Drake, D. J., and McGraw, K. J. (2023). Variation in plasma protein levels in house finches (Haemorhous mexicanus): Effects of season, disease state, and urbanization. Journal of Ornithology 164, 629–638. doi:10.1007/s10336-023-02062-y
- Duca, C., and Marini, MÂ. (2011). Variation in breeding of the Shrike-like Tanager in Central Brazil. The Wilson Journal of Ornithology 123(2), 259–265. doi:10.1676/10-116.1
- Eisermann, K., Arbeiter, S., López, G., Avendaño, C., and De León Lux, J. (2011a). Distribution, habitat use, and implications for the conservation of the globally threatened Azure-Rumped tanager Tangara cabanisi in Guatemala. Bird Conservation International 21, 423–437. doi:10.1017/S0959270910000638
- Eisermann, K., Arbeiter, S., López, G., Avendaño, C., De Lux, J. L., Burge, A., et al. (2011b). Nesting ecology of the endangered Azure-Rumped Tanager (Tangara cabanisi) in Guatemala. Ornitologia Neotropical 22, 39–57. doi:10.2173/nb.azrtan1.01
- Faccio, M. S., Gabriel, V. A., and Pizo, M. A. (2018). Tropical frugivorous birds molt and breed in relation to the availability of food resources. Ornitología Neotropical 29, S11–S18. doi:10.58843/ornneo.v29i2.156
- Gelman, A., and Su, Y. (2022). ‘_arm: Data analysis using regresSion and multilevel/hierarchical models_. R package version 1.13-1.’ Available at https://CRAN.R-project.org/package=arm
- Gentle, L. K., and Gosler, A. G. (2001). Fat reserves and perceived predation risk in the great tit, Parus major. Proceedings of the Royal Society of London, Series B: Biological Sciences 268, 487–491. doi:10.1098/rspb.2000.1405
- Gill, F. (2007). ‘Ornithology,’ 3rd edn. (William Hazen Freeman: New York, NY.)
- Gordon, M., and Lumley, T. (2022). ‘_forestplot: Advanced forest plot using ‘grid’ graphics_. R package version 3.1.1.’ Available at https://CRAN.R-project.org/package=forestplot
- Hidalgo Aranzamendi, N., Hall, M. L., Kingma, S. A., van de Pol, M., Peters, A., and Gill, J. (2019). Rapid plastic breeding response to rain matches peak prey abundance in a tropical savanna bird Ed J Gill. Journal of Animal Ecology 88(11), 1799–1811. doi:10.1111/1365-2656.13068
- Houston, A. I., and McNamara, J. M. (1993). A theoretical investigation of the fat reserves and mortality levels of small birds in winter. Ornis Scandinavica 24, 205. doi:10.2307/3676736
- Hutton, P., McKenna, J., and McGraw, K. J. (2021). Urban links to molt schedule, body condition and carotenoid‐based coloration in the house finch Haemorhous mexicanus. Journal of Avian Biology 52, jav.02761. doi:10.1111/jav.02761
- IBAMA. (1994). ‘Manual de Anilhamento de Aves Silvestres.’ 2nd edn. (Eds I. de L Serrano, J. L. X. do Nascimento and P. de TZ Antas.) (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis: Brasília.)
- IBGE - Instituto Brasileiro de Geografia e estatística. (2013). ‘40 anos de regiões metropolitanas no Brasil.’ (IBGE: Brasília, DF.)
- Ives, A., Dinnage, R., Nell, L., Helmus, M., and Li, D. (2020). ‘_phyr: Model based phylogenetic analysis_. R package version 1.1.0.’ Available at https://CRAN.R-project.org/package=phyr.
- Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., and Mooers, A. O. (2012). The global diversity of birds in space and time. Nature 491(7424), 444–448. doi:10.1038/nature11631
- Jiménez-Peñuela, J., Ferraguti, M., Martínez-de la Puente, J., Soriguer, R., and Figuerola, J. (2019). Urbanization and blood parasite infections affect the body condition of wild birds. Science of the Total Environment 651, 3015–3022. doi:10.1016/j.scitotenv.2018.10.203
- Johnson, E. I., Stouffer, P. C., and Bierregaard, R. O., Jr. (2012). The phenology of molting, breeding and their overlap in central Amazonian birds. Journal of Avian Biology 43(2), 141–154. doi:10.1111/j.1600-048X.2011.05574.x
- Jones, J., Francis, C. M., Drew, M., Fuller, S., and Ng, M. W. S. (2002). Age-related differences in body mass and rates of mass gain of passerines during autumn migratory stopover. The Condor 104, 49–58. doi:10.1093/condor/104.1.49
- Klatt, P. H., Stutchbury, B. J. M., and Evans, M. L. (2008). Incubation feeding by male scarlet tanagers: A mate removal experiment. Journal of Field Ornithology 79(1), 1–10. doi:10.1111/j.1557-9263.2008.00139.x
- Loaiza-Muñoz, M. A., and Londoño, G. A. (2020). Nesting biology of Green-and-gold tanager (Tangara schrankii): Unique traits for lowland reproductive success? Journal of Natural History 54(29–30), 1863–1877. doi:10.1080/00222933.2020.1829725
- Magalhães, V. S., De Azevedo Júnior, S. M., De Lyra-Neves, R. M., Telino-Júnior, W. R., and De Souza, D. P. (2007). Biology of birds captured in an Atlantic Forest fragment at Igarassu, Pernambuco, Brazil. Revista Brasileira de Zoologia 24, 950–964. doi:10.1590/s0101-81752007000400011
- Mainwaring, M. C., and Hartley, I. R. (2013). The energetic costs of nest building in birds. Avian Biology Research 6(1), 12–17. doi:10.3184/175815512X13528994072997
- Marini, M. Â., and Durães, R. (2001). Annual patterns of molt and reproductive activity of passerines in south-central Brazil. The Condor 103(4), 767–775. doi:10.1093/condor/103.4.767
- Martínez, J. E., Zuberogoitia, Í., Calvo, J. F., Álvarez, M., and Margalida, A. (2022). Effect of nest composition, experience and nest quality on nest-building behaviour in the Bonelli’s Eagle. Scientific Reports 12(1), 4146. doi:10.1038/s41598-022-08028-z
- Maute, K. L., French, K., Legge, S., and Astheimer, L. (2013). Seasonal stress physiology and body condition differ among co-occurring tropical finch species. Journal of Comparative Physiology B 183(8), 1023–1037. doi:10.1007/s00360-013-0775-y
- Morellato, L. P. C., Talora, D. C., Takahasi, A., Bencke, C. C., Romera, E. C., and Zipparro, V. B. (2000). Phenology of Atlantic rain forest trees: A comparative study. Biotropica 32(4b), 811–823. doi:10.1111/j.1744-7429.2000.tb00620.x
- Morinay, J., Forsman, J. T., Kivelä, S. M., Gustafsson, L., and Doligez, B. (2018). Heterospecific nest site copying behavior in a wild bird: Assessing the influence of genetics and past experience on a joint breeding phenotype. Frontiers in Ecology and Evolution 5. doi:10.3389/fevo.2017.00167
- Mortensen, J. L., and Reed, J. M. (2018). Parental incubation patterns and the effect of group size in a Neotropical cooperative breeder. The Auk 135(3), 669–692. doi:10.1642/AUK-17-236.1
- Murphy, M. E. (1996). Energetics and nutrition of molt. In ‘Avian Energetics and Nutritional Ecology' (Eds C. Carey.) pp. 543. (Springer: Boston, MA.) doi:10.1007/978-1-4613-0425-8_6
- Nadal, J., Ponz, C., and Margalida, A. (2018). The effects of scaling on age, sex and size relationships in Red-legged Partridges. Scientific Reports 8(1), 1–7. doi:10.1038/s41598-018-20576-x
- Nwaogu, C. J., Dietz, M. W., Tieleman, B. I., and Cresswell, W. (2017). Breeding limits foraging time: Evidence of interrupted foraging response from body mass variation in a tropical environment. Journal of Avian Biology 48(4), 563–569. doi:10.1111/jav.01132
- Oppel, S., Hilton, G. M., Allcorn, R., Fenton, C., Matthews, A. J., Gibbons, D. W., and Stewart, I. (2013). The effects of rainfall on different components of seasonal fecundity in a tropical forest passerine Ed I Stewart. Ibis 155(3), 464–475. doi:10.1111/ibi.12052
- Owen, J. C., Sogge, M. K., and Kern, M. D. (2005). Habitat and sex differences in physiological condition of breeding Southwestern Willow Flycatchers (Empidonax traillii extimus). The Auk 122, 1261–1270. doi:10.1093/auk/122.4.1261
- Peig, J., and Green, A. J. (2010). The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Functional Ecology 24, 1323–1332. doi:10.1111/j.1365-2435.2010.01751.x
- Petrie, A. (2020). ‘Regclass: Tools for an introductory class in regression and modeling. R package version 1.6.’ Available at https://cran.r-project.org/package=regclass
- Podlaszczuk, P., Włodarczyk, R., Janiszewski, T., Kaczmarek, K., and Minias, P. (2017). When moult overlaps migration: Moult-related changes in plasma biochemistry of migrating common snipe. PeerJ 5, e3057. doi:10.7717/peerj.3057
- Pollock, H. S., Brawn, J. D., Cheviron, Z. A., and Williams, T. (2021). Heat tolerances of temperate and tropical birds and their implications for susceptibility to climate warming Ed T Williams. Functional Ecology 35(1), 93–104. doi:10.1111/1365-2435.13693
- Portugal, S. J., Green, J. A., and Butler, P. J. (2007). Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): The importance of wing moult. Journal of Experimental Biology 210(8), 1391–1397. doi:10.1242/jeb.004598
- R Core Team. (2019). ‘R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing: Vienna, Austria.) Available at https://www.R-project.org/
- Redfern, C. P. F. (2010). Brood‐patch development and female body mass in passerines. Ringing & Migration 25(1), 33–41. doi:10.1080/03078698.2010.9674412
- Reif, J., and Štěpánková, K. (2016). Global analysis of threat status reveals higher extinction risk in tropical than in temperate bird sister species. European Journal of Ecology 2, 21–34. doi:10.1515/eje-2016-0003
- Rodrigues, R. C., Hasui, É., Assis, J. C., Pena, J. C. C., Muylaert, R. L., Tonetti, V. R., et al. (2019). Atlantic bird traits: A data set of bird morphological traits from the Atlantic forests of South America. Ecology 100(6), 1–2. doi:10.1002/ecy.2647
- Rodríguez-Ruíz, E. R., Garza-Torres, H. A., Ríos-Muñoz, C. A., and Navarro-Sigüenza, A. G. (2011). The geographical distribution of the blue-gray tanager (Thraupis episcopus) through anthropogenically modified habitats in Mexico. Revista Mexicana de Biodiversidad 82. doi:10.22201/ib.20078706e.2011.3.767
- Rosenberg, K. V., Lowe, J. D., and Dhondt, A. A. (1999). Effects of forest fragmentation on breeding tanagers: A continental perspective. Conservation Biology 13(3), 568–583. doi:10.1046/j.1523-1739.1999.98020.x
- Ruhs, E. C., Martin, L. B., and Downs, C. J. (2020). The impacts of body mass on immune cell concentrations in birds. Proceedings of the Royal Society B: Biological Sciences 287, 20200655. doi:10.1098/rspb.2020.0655
- Saino, N., Romano, M., Caprioli, M., Lardelli, R., Micheloni, P., Scandolara, C., et al. (2013). Molt, feather growth rate and body condition of male and female Barn Swallows. Journal of Ornithology 154(2), 537–547. doi:10.1007/s10336-012-0924-1
- Sánchez-Martínez, M. A., and Londoño, G. A. (2017). Nesting biology of the Ochre‐Breasted tanager (Chlorothraupis Stolzmanni) in Tatamá national natural park, Colombia. Ornitología Neotropical 28, 37–41. doi:10.58843/ornneo.v28i0.104
- Scherer, A. L., Scherer, J. F. M., Petry, M. V., and Victor, H. (2014). Sexual dimorphism and body condition of wintering White-rumped Sandpipers in southern Brazil. Wilson Journal of Ornithology 126, 553–561. doi:10.1676/13-121.1
- Şekercioğlu, Ç. H., Primack, R. B., and Wormworth, J. (2012). The effects of climate change on tropical birds. Biological Conservation 148(1), 1–18. doi:10.1016/j.biocon.2011.10.019
- Slagsvold, T., and Wiebe, K. L. (2011). Social learning in birds and its role in shaping a foraging niche. Philosophical Transactions of the Royal Society B: Biological Sciences 366(1567), 969–977. doi:10.1098/rstb.2010.0343
- Smith, P. A., Gilchrist, H. G., and Smith, J. N. M. (2007). Effects of nest habitat, food, and parental behavior on shorebird nest success. The Condor 109, 15–31. doi:10.1093/condor/109.1.15
- Snchez Martínez, M. A., and Londoño, G. A. (2012). First nesting information for the orange-eared tanager (Chlorochrysa calliparea). Wilson Journal of Ornithology 124, 380–384. doi:10.1676/11-046.1
- Souza Penha, V. A., and da Silva Rodrigues, R. (2022). Sex, age, mean annual temperature and year predict the body condition in Chiroxiphia caudata (Passeriformes: Pipridae). Journal of Ornithology 163, 445–456. doi:10.1007/s10336-021-01947-0
- Souza, A. E. B. A., and Serafini, P. P. (2020). ‘Manual de Anilhamento de Aves Silvestres,' 3rd edn. (ICMBio, Cemave: Brasilia.)
- Swaddle, J. P., and Witter, M. S. (1997). The effects of molt on the flight performance, body mass, and behavior of European starlings (Sturnus vulgaris): An experimental approach. Canadian Journal of Zoology 75(7), 1135–1146. doi:10.1139/z97-136
- Veloso, S. L., Pesquero, M. A., Rodrigues, L. G., and Pesquero, M. F. (2018). Parental care of the swallow tanager (Tersina viridis) in southern goiás, Brazil. The Wilson Journal of Ornithology 130(3), 658–663. doi:10.1676/17-063.1
- Wickham, H. (2016). ‘Ggplot2: Elegant Graphics for Data Analysis (Use R).’ (Springer: Verlag, New York.)
- Williams, T. D. (2018). Physiology, activity and costs of parental care in birds. Journal of Experimental Biology 221(17). doi:10.1242/jeb.169433
- Winkler, D. W., Billerman, S. M., and Lovette, I. J. (2020). Tanagers and allies (Thraupidae). In ‘Birds of the World.’ (Eds S. Billerman, B. Keeney, P. Rodewald, and T. Schulenberg.) (Cornell Lab of Ornithology.) doi:10.2173/bow.thraup2.01
- Wojczulanis-Jakubas, K., Jakubas, D., Chastel, O., and Kulaszewicz, I. (2015). A big storm in a small body: Seasonal changes in body mass, hormone concentrations and leukocyte profile in the little auk (Alle alle). Polar Biology 38(8), 1203–1212. doi:10.1007/s00300-015-1687-y
- Wolfe, J. D., Pyle, P., and Ralph, C. J. (2009). Breeding seasons, molt patterns, and gender and age criteria for selected northeastern Costa Rican resident landbirds. The Wilson Journal of Ornithology 121(3), 556–567. doi:10.1676/08-111.1
- Wolfe, J. D., Ryder, T. B., and Pyle, P. (2010). Using molt cycles to categorize an integrative new system. Journal of Field Ornithology 81, 186–194. doi:10.1111/j.1557-9263.2010.00276.x
- Wolfe, J. D., Ryder, T. B., Pyle, P., and Johnson, E. I. (2012). Using molt and plumage cycles to age tropical: Up-dates and recent advances. Ornitologia Neotropical 23, 169–174.

