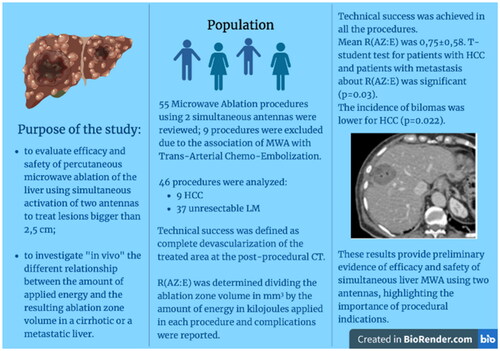
Abstract
Background
sequential or simultaneous applications of multiple antennas have been proposed to create larger ablation zone; however, there is a lack of data in patients affected by liver tumors, with potentially different results from animal liver models. The purpose of this study was to evaluate efficacy and safety of liver percutaneous microwave ablation using simultaneous activation of two antennas to treat lesions bigger than 2,5 cm; particularly the focus was assessing whether the ratio of ablation zone volume in millimeters to applied energy in kilojoules [R(AZ:E)] differs between hepatocellular carcinoma in a cirrhotic liver and liver metastasis and if it is correlated to complications incidence or recurrence of disease.
Methods
Fifty-five liver microwave ablation performed with two simultaneous antennas from March 2017 to June 2021 were retrospectively reviewed; 9 procedures were excluded due to the association with Chemoembolization. Size, shape, volume of lesions and ablation zones were recorded. Technical success was defined as complete devascularization of the treated area at the post-procedural CT. R(AZ:E) was determined dividing the ablation zone volume in mm3 by the amount of energy in kilojoules applied in each procedure and complications were reported.
Results
Technical success was achieved in all the procedures. Mean R(AZ:E) was 0,75 ± 0,58. T-student test for patients with HCC and patients with metastasis about R(AZ:E) was significant (p = 0.03). The incidence of bilomas was lower for HCC (p = 0.022). One-month follow-up showed Complete Response (CR) in 44/46 (95,6%) patients; Three-six months follow-up demonstrated: CR in 43/46 (93.5%) cases and 12 months follow-up highlighted CR in 40/45 (88,9%) cases.
Conclusions
These results provide preliminary evidence of efficacy and safety of simultaneous liver MWA using two antennas, highlighting the importance of procedural indications.
1. Introduction
For over 20 years, hepatic malignancies have been effectively treated with radiofrequency ablation (RFA) and microwave ablation (MWA) [Citation1]. During a percutaneous ablative treatment, it is crucial avoiding recurrence of disease at the ablation site to guarantee complete local tumor control (A0 ablation). Independent risk factors for incomplete ablation and subsequent tumor recurrence are larger lesion size, proximity to vessels, improper placement of the ablation probe and, most of all, inadequate safety margin around the target lesion. [Citation1,Citation2]. Consequently, several laboratory studies, performed in ex vivo and in vivo animal liver models, proved that a more successful strategy to create larger ablation zone is MWA with simultaneous applications of multiple antennas rather than sequential MWA [Citation3–9]. However, although there is a substantial lack of data about this new treatment modality in patients affected by liver tumors, it is likely that the resulting ablation zones in vivo would differ significantly from animal liver models [Citation10–13]. Besides, the creation of a predictable ablation zone is vital to create a proper safety margin [Citation14,Citation15]. Companies provide physicians with ablation protocols mostly built on ‘ex vivo’ procedures of non-perfused, non-diseased animals’ livers, hence it may occur that the ablation zones obtained in these livers would differ considerably from the one obtained in procedures of diseased livers in humans [Citation2,Citation16]. Studies examining the multi-probes reproducibility of MWA in different tumor types and in abnormal underlying liver parenchyma (cirrhosis) in humans are lacking. Therefore, the main aim of this study was to retrospectively evaluate efficacy and safety of percutaneous MWA performed using simultaneous activation of two antennas for the treatment of liver malignant lesions. Afterwards, another goal was to investigate the relationship between the amount of applied energy and the resulting ablation zone volume, assessing whether the ratio of ablation zone volume in millimeters to applied energy in kilojoules [R(AZ:E)] [Citation1] differs between hepatocellular carcinoma (HCC) in a cirrhotic liver and liver metastasis (LM) and if it is correlated to the complications incidence or recurrence of disease.
2. Materials and methods
This retrospective study was approved by the institutional review board with permission to perform chart review and a waiver of written informed consent. Inclusion criteria for the retrospective analysis were as follows: Patients who underwent percutaneous MWA with the simultaneous activation of two antennas for either LM or HCC with a maximum diameter >2,5 cm from March 2017 to June 2021. All patients were previously discussed in a tumor board meeting in which the decision for MWA was made. Exclusion criteria for the statistical analysis were the association of MWA with Trans-Arterial Chemo-Embolization or with any other endovascular approach in the same session and the absence of a 12-months follow-up .
2.1. Percutaneous MWA
All procedures were performed in deep sedation using US/CT-guidance, with Ropivacaine (5 mg) injected locally on the hepatic capsule; two 15 G antennas spacing ≤2.0 cm were employed with a commercially available MWA system (PR probe - NEUWAVE™; Johnson & Johnson), and manufacturers’ protocols were followed. MWA was performed by simultaneous activation of all the antennas (minimum heating cycle, 65 W; 10 min). Hydro-dissection was utilized to protect nearby non-target structures if needed.
Technical success was defined as complete devascularization of the treated area at the contrast enhanced CT scan performed at the end of the procedure.
2.2. Data collection
The following data were collected: patient features (age, sex); tumor histology, location in liver segments, size, shape (roundness index) and volume (evaluated using the semiautomatic Lesion tool, Vue PACS, Carestream) of both lesions and ablation zones, energy (Watts) and duration (minutes) of heating cycles. The size of the ablation zone was obtained by measuring the long diameter (Dl, along the antenna axis), the short diameter (Ds, perpendicular to the antenna axis), and the vertical diameter (Dv, vertical to Dl and Ds) on immediate post-ablation contrast-enhanced CT. The shape of the lesion and the ablation zone were evaluated through roundness index by calculating the following ratio: Dl/Dv (i.e. values close to 1 were considered spherical). The volume of the ablation zone was calculated on immediate post-ablation contrast-enhanced CT on the portal venous phase acquisition with 2-mm slice reconstruction through the same segmentation method; this tool automatically delineates the tumor volume after an initial manual evaluation of the Diameter of the lesion.
R(AZ:E) was determined by dividing the ablation zone volume in mm3 by the amount of energy in kilojoules applied in each procedure. Complications were recorded during follow-up and categorized according to the CIRSE classification system [Citation17].
Additionally, Patient’s oncological response was assessed according to mRECIST criteria [Citation18] for HCC and RECIST1.1 criteria [Citation19] for other malignancies. We evaluated the Local Tumor Control (LTC) the overall survival (OS) and complications incidence over a minimum follow-up period of 12 months, performing contrast enhanced CT or MR at 1, 3, 6 and 12 months.
2.3. Statistical analysis
A descriptive analysis of continuous variables (volume lesion, volume of the ablated area, Ds, Dl, Dv, R(AZ:E), roundness index, LTC and OS and categoric variables (histology of lesions) was performed. The T test was used to compare the volume of the ablated area, roundness index, R(AZ:E), LTC and OS between patients with HCC and patients with LM. Besides, a subgroup analysis was performed among patients who already underwent liver surgery or a local treatment (i.e. thermal-ablation or radiotherapy) in terms of complications rate and local or systemic recurrence incidence. Statistical significance was set at p ≤ 0.05. The statistical analysis was performed with IBM SPSS statistics version 27.
3. Results
Fifty-five microwave ablations using two simultaneous antennas procedures of liver malignant lesions were performed; 9 procedures were excluded due to the combination of MWA with Trans-Arterial Chemo-Embolization in the same session (). A total of 46 patients were included, 20 male and 26 female. Extensive patients and tumor features are summarized in . A total of 9 HCC and 37 unresectable LM were treated; of the metastases, 15 were recurrences (i.e. 8 cases of previous surgery, 6 case of percutaneous ablation and 1 case of radiotherapy).
Figure 1. Flowchart illustrates number of patients treated with MWA, excluded patients, and subgroup population.

Table 1. Distribution of discrete variables of the patients’ features (age, sex); tumor histology, location in liver segments, size, and volume of both lesions and ablation zones.
Mean lesion Dl was 4 ± 1,16 cm (2,1–7 cm), mean Ds was 3,02 ± 1,02 cm (1,2-6 cm) and mean Dv was 3,43 ± 0,94 cm (1,8–5,7 cm). Mean ablated area Dl was 5,75 ± 1,31 cm (3,6–9 cm), mean Ds was 4,21 ± 1,26 cm (2,3–8 cm) and mean Dv was 4,93 ± 1,18 cm (2,7–7,7 cm). Mean lesion volume was 24,99 ± 20,74 cm3 (5,4–85 cm3) and mean volume of the ablated area was 61,54 ± 42,61 cm3 (10 - 222 cm3). Comparing the lesion volume with the ablated volume, the second one was significantly higher (p < 0.01), while the difference between ablated volume in HCC and in LM was not statistically significant (mean 350 vs 353, p = 0.9).
Moreover, 100% of technical success was achieved in all treated liver malignant lesions. All ablative zones were spherical or ellipsoid with antennas spacing ≤2.0 cm (mean roundness index of the ablation was 1,06 for HCC and 1,23 for LM). Artificial dissection was performed in 8 cases due to diaphragm, liver hilum or colon proximity.
HCC lesions and the associated ablated areas tended to be more spherical (roundness index respectively of 1,08 and 1,06) than LM (1,21 and 1,23), without a statistically significant difference (p = 0,128 and p = 0,111) ().
Mean R(AZ:E) was 0,75 ± 0,58 (2,6 − 0,08); T-student test for patients with HCC and patients with metastasis about R(AZ:E) was significant (p = 0.03) with t=-1.776, with a mean difference of −0.038 (). Between patients who previously already underwent liver surgery or a local treatment (i.e. thermal-ablation or radiotherapy) and patients not treated a significant difference about R(AZ:E) (p = 0.044) was found, with t=−2.186 and a mean difference of −0.091 (). Besides, there were no differences about R(AZ:E) between patients with and without complications in general (p = 0.751), with and without bilomas (p = 0.501) or with and without recurrence (p = 0.566).
In 9 cases (19%) peri-procedural complications were observed: 1 (2,17%) subcapsular hemorrhage (), 7 (15%) cases of bilomas () and 1 (2,17%) peripheral portal thrombosis. Ablated HCC lesions developed bilomas in statistically fewer cases than metastases (T = −2.44, p = 0.022, mean 0 vs 0.19). Moreover, all the patients without complications had a significant lower incidence of previous treatments (p < 0.05).
Figure 5. 78yo with liver metastasis of renal cell carcinoma treated with two microwave antennas. (a, b) US images highlighting the distance between the two probes and the hyperechoic bubbles during the ablation (c) Contrast-enhanced US exam displaying the complete devascularization of the lesion at the end of the procedure. (d) Unenhanced, (e) arterial and (f) portal CT scan images performed at the end of the procedure showing a subcapsular hemorrhage (arrow). (g) Cauterization of hepatic capsular access at 90° for 3 min was performed (circle). (h) Good local bleeding control at the CT scan after 48 h.

Figure 6. 70 yo with liver metastasis of pancreas adenocarcinoma, treated with MWA. (a) portal phase CT scan, coronal plane; (b) intraprocedural ecographic image of the lesion (c) portal phase coronal and axial CT scan showing necrosis in the ablated zone, without evidence of residual disease; small air bubbles (arrow) are present, a common finding after the procedure, which normally disappear during follow-up. (d) portal phase MR performed one month after the ablation revealing an asymptomatic biloma; (e) US examination of the abdomen was performed 3 months after the ablation due to fever, revealing an infected biloma, treated with a percutaneous drainage tube (f, g, h).

3.1. Oncological results
One-month follow-up showed Complete Response (CR) in 44/46 (95,6%) patients, with no residual disease for HCC and 2 cases (5,4%) of residual disease for LM (). Lesions with Partial Response (PR) were a metastasis from colon adenocarcinoma with a diameter of 5,7 cm and a metastasis from breast adenocarcinoma with a diameter of 2,8, which is probably due to one of the two probes malfunction occurred during the procedure. Both the patients were treated with a single probe MWA, achieving CR at the next follow-up.
Table 2. mRECIST and RECIST 1.1 categorization at 1,6 months and then 1,2,3 years for HCC and for LM, with percentage of Complete Response (CR), Partial Response (PR) and Progression Disease (PD) at each time point.
Three-six months follow-up demonstrated: CR in 43/46 (93.5%) cases (among these patients were the two who had obtained PR at first follow-up and were successfully retreated), PR in 2/37 (9.5%) and a progression of disease (PD) in 1/37 patients (3.7%). Both the patients classified as PR had a metastasis from colon adenocarcinoma: respectively a 4,6 cm lesion in segments 3–4, which was then successfully treated with segmentectomy thanks to the tumor debulking, and a 5 cm lesion in segment 5, treated with a new single probe MWA, achieving CR at the next follow-up. The PD was reported in a patient with a diffuse metastatic infiltration from colon adenocarcinoma (with a diameter of the treated lesion of 5,6 cm), and the patient died 8 months after the procedure due to heart failure.
Twelve months follow-up highlighted CR in 40/45 (88,9%) cases and Three cases (8,6%) of PR, respectively a LM from gastric adenocarcinoma (Segment 6–7 lesion, 3,7 cm Dl), breast adenocarcinoma (Segment 4 lesion, 2,8 cm Dl) and colon adenocarcinoma (Segment 8 lesion, 5,7 cm Dl). The first two cases had a multinodular liver recurrence and was treated with TACE, while the other patient was treated with hepatic cryoablation due to the proximity of the recurrence to the diaphragm. One patient with HCC reported a progression of disease at 12 months and was treated with TACE; one patient with LM from esophagus adenocarcinoma presented diffuse liver and lung progression and died 14 months after the treatment.
In all our cohort, mean LTC for HCC was 22,5 ± 11,5 and for LM was 20,1 ± 8,9, while the OS was 16,7 ± 8,9 for HCC and 14,1 ± 8,2 for LM, without a statistically significant difference between the two groups.
4. Discussion
The retrospective analysis confirmed ‘in vivo’ the expected results of the efficacy of MWA using two simultaneous antennas. Mean axial Diameter of the ablated area was 5,75 ± 1,31 cm, which is nearly 1,5 cm larger than the mean axial Diameter of the lesion (4 ± 1,16 cm), thus indicating an acceptable oversizing of the ablation area. This impression was further corroborated by the volumetric examination, which showed that the mean lesion volume was 24,99 ± 20,74 cm3 (5,4 – 85 cm3) and mean volume of the ablated area was 61,54 ± 42,61 cm3 (10 – 222 cm3), at least double than the lesion volume. These results validate in a larger cohort of patients that the use of simultaneous multi-antenna MWA applied to treat liver tumors results in large, nearly spherical ablation volumes, as presented by Cazzato et al. [Citation5] and Garnon et al. [Citation6].
HCC lesion and the associated ablated area tended to be more spherical (roundness index respectively of 1,08 and 1,06) than LM (1,21 and 1,23) but without a statistically significant difference (p = 0,128 and p = 0,111). Nonetheless, these data reveal how multi-probe MWA offers the chance to create a tailored ablation area, reproducing the shape of the lesion with an adequate safe margin. Vogl et al. [Citation20] have demonstrated that spherical ablations result in larger ablative margins. Hence, MWA ablation using two probes ensures a larger ‘safe margin’, providing a reliable mean of reaching the A0 target of ablation, even for bigger lesions ( and ). For this reason, new MWA devices are designed to achieve spherical ablation morphology. There are different reports about the efficacy of the single-antenna approach for tumors sized less than 3 cm [Citation21,Citation22]; on the other hand, as highlighted by Cazzato et al. [Citation5] and confirmed by our experience, a multi-antenna approach also for 2–3-cm-sized tumors may ensure a better LTC.
Figure 7. 74 year old woman, with liver recurrence of breast cancer of 36 x 36 mm(a). (b) The lesion was treated with simultaneous activation of two PR antennas; single treatment with a power of 65 W for 10 min was performed. (c, d, e) MIP and MRP reconstructions of the immediate post-procedural CT showing an ellipsoid ablative zone of 60 x 43 x60 mm with complete devascularization.

Figure 8. Evolution of the ablation zone from the previous case, showing no evidence of recurrence 10 months after the ablation.

T student test for patients with HCC and patients with metastasis about the ratio of ablation zone volume in mm3 to applied energy in kilojoules [R(AZ:E)] is significant (p = 0.03) with a mean difference of −0.038. This result shows that [R(AZ:E)] was higher for HCC and hypothetically suggests that, with the same number of Joules delivered during the procedure, the ablation zone will be larger treating a HCC in a cirrhotic liver than a LM. This is probably due to a difference in tissue characteristics between normal and cirrhotic liver parenchyma and a modification in total liver blood flow between normal livers (with LM) and cirrhotic livers (with HCC): indeed, in most studies, cirrhotic liver parenchyma has been found less perfused than normal liver parenchyma (Citation23), resulting in an increase in ablation zone volume (Citation24). Another possible explanation for dissimilarities in energy deposition between HCC and LM could also be represented by the pseudocapsule of HCCs, which has been identified as a cause of an hypothetical ‘oven effect’, phenomenon characterized by higher temperatures inside the tumor and lower temperatures outside due to the low thermal conductivity of the capsule. This is also the possible reason why ablated HCC lesions have bilomas in statistically fewer cases than metastases (T = −2.44, p = 0.022, mean 0 vs 0.19).
Besides, between patients who previously already underwent liver surgery or a local treatment and those not treated, a significant difference about [R(AZ:E)] (p = 0.044) was found, with a mean difference of −0.091, thus implying a more aggressive approach of the operator when treating a recurrence to obtain a bigger ‘safe margin’. On the other hand, when analyzing the dissimilarities in terms of [R(AZ:E)] between patients with and without complications (p = 0.751), no statistically significant difference was highlighted. Hence, performing longer ablations or using high Wattages did not affect the complications incidence: these findings confirm the preliminary results of Cazzato et al. highlighting that the large ablation zones achieved were not obtained at the cost of an increased morbidity [Citation5].
Therefore, this analysis provides evidence of the safety of multi-probe MWA, with just minor complications incidence: the subcapsular hemorrhage () occurred at the level of one of the two probes insertion, a common risk when performing MWA, and was promptly treated ‘cauterizing’ the probe path (grade 3 of the CIRSE Classification System for Complications), the peripheral portal thrombosis was treated with EBPM and resolved after 4 months (grade 3) and out of 7 cases of bilomas (grade 3), only one case relapsed after percutaneous drainage and required the permanent positioning of a biliary drainage (thus upgraded at grade 4).
Furthermore, another interesting result was that all the patients who had complications have had a significant higher incidence of previous treatments (p < 0.05), especially for the cases of biloma: among the 7 patients with this complication, 5 had already underwent a liver procedure (i.e. 3 cases of previous surgery, 1 case of percutaneous ablation and 1 case of radiotherapy) and that ablated HCC lesions had bilomas in statistically fewer cases than metastases (T = −2.44, p = 0.022, mean 0 vs 0.19). These data may be explained by the cytoarchitectural alteration of the liver subsequent to the local treatment, and suggest that performing double-probe MWA on patient who did not already undergo a surgical or percutaneous procedure on the liver would be a safer option, especially when treating a LM.
Nevertheless, 9 patients were excluded from the analysis due to the association of MWA and TACE in the same session; the combined treatment was used predominantly for juxta-diaphragmatic lesions, especially in the left lobe, where maximal MW power was not used to avoid non-target injuries, i.e. diaphragmatic or epicardial damages. A possible different choice for these kind of lesions could be the ‘Percutaneous Thermal Segmentectomy’: Lucatelli et al. [Citation25], highlighted how the combination of balloon-occluded MWA followed by balloon-occluded TACE supply a great volume of ablation (75.0 cm3). It is worth stating that the multiple antenna procedures need accurate geometrical planning and positioning, all technical aspects that may be difficult for subcapsular lesions [Citation26]. On the other hand, Percutaneous Thermal Segmentectomy is performed mainly with a single antenna, with no need of accurate geometrical planning but just positioning within the lesion as standard.
This study is hindered by some limitations, i.e., the retrospective nature of the evaluation and the absence of multi-center involvement and randomization. New prospective studies about multi-probe MW liver ablation involving different centers are needed, to confirm the results obtained in this cohort of patients.
5. Conclusions
These results provide preliminary evidence of efficacy of simultaneous MWA using two antennas to treat liver malignant lesions, in terms of both creating a larger necrotic area and ensuring higher safety (i.e., low complication rate), highlighting the necessity of other studies to properly inquire the differences of this approach for the treatment of HCC and LM.
Institutional review board statement
This study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Campus Bio-Medico University of Rome (Prot. N° 01/19 OSS ComEt CBM).
Informed consent statement
Patient informed consent was not necessary according to the retrospective nature of the study and after approval of our Ethics Committee Approval.
Acknowledgments
The graphical abstract of this manuscript was created using BioRender.
Disclosure statement
No potential conflict of interest was reported by the author(s). The APC funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data availability statement
Not applicable.
Additional information
Funding
References
- Heerink WJ, Solouki AM, Vliegenthart R, et al. The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis. Eur Radiol. 2018;28(8):3228–3236.
- Ruiter SJS, Heerink WJ, de Jong KP. Liver microwave ablation: a systematic review of various FDA-approved systems. Eur Radiol. 2019;29(8):4026–4035.
- Harari CM, Magagna M, Bedoya M, et al. Microwave ablation: comparison of simultaneous and sequential activation of multiple antennas in liver model systems. Radiology. 2016;278(1):95–103.
- Zhang TQ, Huang SM, Gu YK, et al. Sequential and simultaneous 4-antenna microwave ablation in an ex vivo bovine liver model. Cardiovasc Intervent Radiol. 2019;42(10):1466–1474.
- Cazzato RL, De Marini P, Leclerc L, et al. Large nearly spherical ablation zones are achieved with simultaneous multi-antenna microwave ablation applied to treat liver tumours. Eur Radiol. 2020;30(2):971–975.
- Garnon J, Delmas L, De Marini P, et al. Triple-Antenna microwave ablation with repositioning for the creation of a reliable 6-cm ablation zone in the liver. Cardiovasc Intervent Radiol. 2021;44(8):1291–1295.
- Amabile C, Ahmed M, Solbiati L, et al. Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical haracterization. Int J Hyperthermia. 2017;33:34–42.
- Hines-Peralta AU, Pirani N, Clegg P, et al. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239(1):94–102.
- Wright AS, Lee FT, Jr, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10(3):275–283.
- Lu DSK, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14(10):1267–1274.
- Ringe KI, Lutat C, Rieder C, et al. Experimental evaluation of the heat sink effect in hepatic microwave ablation. PloS One. 2015;10(7):e0134301.
- Winokur RS, Du JY, Pua BB, et al. Characterization of in vivo ablation zones following percutaneous microwave ablation of the liver with two commercially available devices: are manufacturer published reference values useful? J Vasc Interv Radiol. 2014;25(12):1939–1946.e1.
- Dodd GD, 3rd, Kreidler SM, Lanctot AC, et al. Effect of change in portal venous blood flow rates on the performance of a 2.45-GHz microwave ablation device. Radiology. 2015;277(3):727–732.
- Ahmed M, Solbiati L, Brace CL, For the International Working Group on Image-guided Tumor Ablation, Interventional Oncology Sans Frontières Expert Panel, Technology Assessment Committee of the Society of Interventional Radiology, and the Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Deshazer G, Merck D, Hagmann M, et al. Physical modeling of microwave ablation zone clinical margin variance. Med Phys. 2016;43(4):1764.
- Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38(2):61–67.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assess- ment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline. Eur J Cancer. 2009;45(2):228–247.
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–1146.
- Vogl TJ, Basten LM, Nour-Eldin NA, et al. Evaluation of microwave ablation of liver malignancy with enabled constant spatial energy control to achieve a predictable spherical ablation zone. Int J Hyperthermia. 2018;34(4):492–500.
- Saccomandi P, Schena E, Massaroni C, et al. Temperature monitoring during microwave ablation in ex vivo porcine livers. Eur J Surg Oncol. 2015;41(12):1699–1705.
- Groeschl RT, Pilgrim CH, Hanna EM, et al. Microwave ablation for hepatic malignancies: a multi-institutional analysis. Ann Surg. 2014;259(6):1195–1200.
- Van Beers BE, Leconte I, Materne R, et al. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol. 2001;176(3):667–673.
- Hashimoto K, Murakami T, Dono K, et al. Assessment of the severity of liver disease and fibrotic change: the usefulness of hepatic CT perfusion imaging. Oncol Rep. 2006;16(4):677–683.
- Lucatelli P, Argirò R, Crocetti L, et al. Percutaneous thermal segmentectomy: proof of concept. Cardiovasc Intervent Radiol. 2022 May;45(5):665–676.
- Vo Chieu VD, Werncke T, Hensen B, et al. CT-Guided microwave ablation of liver tumors in anatomically challenging locations. Cardiovasc Intervent Radiol. 2018;41(10):1520–1529.