Abstract
Purpose: To investigate the feasibility rate and the mid-term outcomes of fusion imaging-guided radiofrequency ablation (RFA) with artificial ascites or pleural effusion of hepatocellular carcinomas (HCCs) based on tumor locations.
Materials and Methods: In this single-center retrospective study, 456 patients with single HCCs ≤4 cm were referred for RFA from April 2019 to April 2020. The tumor locations were classified into a conventional location (CL) and difficult location (DL, close to the diaphragm/heart/major vessels/bile ducts/gastrointestinal tract/kidneys). This study assessed the feasibility rate of CT/MRI-US fusion system-guided RFA with artificial ascites or pleural effusion and the therapeutic outcomes including technical success, technique efficacy, and local tumor progression (LTP) according to tumor location. Cumulative LTP rates were estimated using the Kaplan–Meier method.
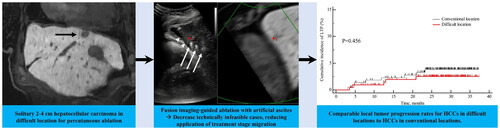
Results: 235 of 456 (51.5%) patients had HCCs in DL. Ablation was feasible in 431 of 456 (94.5%) patients. The feasibility rate was significantly lower in DL group than in CL group (89.8% [211/235] vs. 99.5% [220/221], p < 0.001). The technical success and technique efficacy rates were 100% [211/211] vs. 99.5% [219/220] and 98.6% [208/211] vs. 100% [220/220] in DL and CL groups, respectively (p > 0.05). The estimated 1-, 2-, and 3-year cumulative LTP rates in DL group were 1.0%, 2.5%, and 2.5%, respectively, and were not significantly different from the 2.3%, 3.9%, and 3.9% observed in CL group (p = 0.456).
Conclusion: Fusion imaging-guided RFA with artificial ascites or pleural effusion could decrease technically infeasible cases and provide comparable LTP rates for HCCs in DL to HCCs in CL.
Introduction
Percutaneous ablation is considered one of the main curative treatments for hepatocellular carcinomas (HCCs) <5 cm that develops in cirrhotic livers, along with surgical resection and liver transplantation [Citation1]. In patients with early-stage HCC (single, or ≤3 HCCs each ≤3 cm), ablation could be prioritized if there is a contraindication of liver transplantation or clinically significant portal hypertension [Citation2,Citation3]. The updated Barcelona Clinic Liver Cancer (BCLC) algorithm [Citation2] and the European Association for the Study of the Liver (EASL) guideline [Citation3] introduce the concept of treatment stage migration (TSM) strategy (i.e., treatment recommendations that would usually be considered for a more advanced stage) and frequently recommend transarterial chemoembolization (TACE). In other words, if percutaneous ablation is infeasible in BCLC 0 or A patients, patients may receive palliative treatment such as TACE rather than a curative treatment option [Citation2,Citation3]. Regarding the feasibility of percutaneous ablation, the location of the HCC is a critical factor. When HCCs are located in difficult locations (hepatic dome, caudate lobe, close to the major intrahepatic vessels, bowel, gallbladder, colon, and heart), these tumors are more challenging to treat with ablation and may limit their feasibility [Citation4,Citation5]. Furthermore, those tumors are more prone to residual disease or local tumor progression (LTP) after ablation [Citation1,Citation6,Citation7].
To overcome these problems, several approaches can be combined, such as multimodality-ultrasound (US) fusion guidance, artificial ascites and pleural effusions, CT guidance, or more powerful energy sources such as high-power microwave ablation (MWA) or multi-applicator radiofrequency ablation (RFA) [Citation8–13]. Although several studies have demonstrated that CT-guided MWA may improve local tumor control rates for subdiaphragmatic tumors compared with US-guided RFA [Citation8–11], it is still challenging to position ablation devices with more inclined approaches on CT once the patient is deeply sedated and collateral damage may occur [Citation6]. In Asia and some countries in other continents, accessibility to CT for guiding ablation procedures is limited due to the high volume of diagnostic CT examinations, and US is the most common guiding modality for ablation procedures [Citation14,Citation15]. Multimodality-US fusion imaging not only helps more accurately determine the target lesion by enabling ablation of poorly visible or invisible tumors but also improves the monitoring of procedures [Citation14]. The use of artificial ascites was helpful in minimizing collateral thermal injury and improving the sonic window for ablation of HCCs abutting the diaphragm or gastrointestinal tract [Citation16]. However, previous studies [Citation8–12,Citation16] regarding ablation for HCCs in difficult locations are limited in terms of small patient samples, and a systematic analysis regarding the infeasibility of percutaneous ablation for small HCCs based on tumor locations and the prevalence of TSM strategy application would be necessary.
Therefore, in this study, we attempted to investigate the feasibility rate and mid-term outcomes of CT/MRI-US fusion system-guided RFA with artificial ascites or effusion of HCCs based on tumor locations.
Materials and methods
This single-center retrospective study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 2207-082-1139). Therefore, the need for written informed consent was waived.
Patients
A total of 492 patients with 526 HCCs were referred to the radiology department for percutaneous ablation from April 2019 to April 2020. All HCCs were diagnosed by histopathology or noninvasive imaging-based diagnosis according to the Korean Liver Cancer Association-National Cancer Center Korea guidelines [Citation17]. The inclusion criteria for this study were as follows: a) single HCC, 1.0–4.0 cm; b) contrast-enhanced CT or MRI within 60 days before ablation; c) Child-Pugh class A or B liver function; and d) age, 20–85 years. The exclusion criteria were as follows: a) single HCC >4 cm or multiple HCCs; b) presence of macrovascular invasion and/or distant metastasis; c) platelet count <50,000 mm3, or international normalized ratio >1.5 (prothrombin time >1.5 × normal); d) Child-Pugh class C liver function; and d) follow-up period <3 months after treatment. Among the 492 patients, 36 were excluded for the following reasons: a) multiple HCCs (n = 30), b) Child-Pugh class C (n = 1), and c) follow-up period <3 months (n = 5). Therefore, the remaining 456 patients with a single HCC constituted the study population (). Ablation planning US, pretreatment CT, and/or MRI were reviewed to evaluate tumor locations and ablation feasibility rates.
Definition of difficult locations
Based on previous studies [Citation6,Citation8–11,Citation13,Citation16,Citation18–20], we defined difficult locations of HCCs for ablation in axial or coronal images of pretreatment CT or MRI as follows: a) lesions adjacent to large vessels (≤5 mm from the first or second branch of the portal vein, the base of the hepatic vein, or the inferior vena cava); b) lesions adjacent to (≤5 mm) the 1st or 2nd branch bile duct; c) lesions located ≤5 mm from the diaphragm, gallbladder, right kidney, or gastrointestinal tract; and d) lesions located ≤1 cm from the pericardium.
Ablation procedures and strategies for HCCs in difficult locations
All ablation procedures were performed under local anesthesia and conscious sedation by one of the four radiologists with at least 5 years of experience in image-guided ablation of HCC. Real-time CT/MRI-US fusion imaging (R.S. 85, Samsung Medison) was used as the guidance modality. A separable clustered applicator with three applicators (Octopus radiofrequency [RF] applicator, STARmed) and a multichannel RF generator (VIVA RF System, STARmed) was used. The number and active needle tip length of the applicators were chosen based on the tumor size, as described in our previous study [Citation21]. In general, when the tumor size was <1.5 cm, two or three applicators with 2-cm active tips were selected, and three applicators with 2.5- or 3-cm active tips were preferentially used for 1.5–5.0 cm-sized tumors. For in situ monitoring of the ablation procedure, a virtual tumor marker on real-time US-CT/MRI fusion imaging was used to determine whether the ablation zone showing hyperechogenicity covered the index tumor and whether the ablation zone approached adjacent vital structures during the procedure.
Strategies for HCCs in difficult locations
For HCCs adjacent to the diaphragm, gallbladder, right kidney, gastrointestinal tract, or pericardium, artificial ascites (5% dextrose in a water solution [5% DW]) was injected into the perihepatic space using a 5 F angiosheath with several additional side holes. Artificial pleural effusion was also created for tumors in segments VII or VIII near the diaphragm to secure a window for tumor visualization and to prevent lung injury. The puncture sites for introducing artificial ascites were selected according to tumor location. The right intercostal space along the anterior axillary line was used to treat the tumors in the right hepatic lobe. In contrast, the left epigastric area was selected for tumors in segments II, III, and IV. We attempted to separate the ablation zone to at least 1 cm from the adjacent organ using 500 ml to 2,000 ml 5% DW.
When an index tumor abutted the right colon or gallbladder, the tip of a 5 F angiosheath was placed between the index tumor and those organs. In patients with a history of prior treatment (surgery or transarterial chemoembolization), sufficient artificial ascites may not be infused between the liver and adjacent organs owing to perihepatic adhesions. In this case, we placed a 5 F angiosheath or 18-gauge spinal needle between the liver and adjacent organs; thereafter, 500 ml to 1,000 ml of 5% DW has manually injected to hydro dissect the adhesion [Citation12].
For tumors abutting intrahepatic vessels (<5 mm in diameter), we attempted to overcome the heat sink effect by inserting applicators between the tumor and those vessels, and RF energy was delivered using a combination of switching monopolar and bipolar modes [Citation22]. In the case of centrally located tumors adjacent to large bile ducts (≤5 mm apart from the 1st or 2nd order branch), ablation was not performed to avoid serious complications due to bile duct injury. However, if the tumor was located in the periphery within 4 cm of the liver capsule and the adjacent bile duct was a segmental branch, ablation was performed while trying to avoid bile duct injury using the real-time US fusion imaging technique.
Technical success, technique efficacy, local control, and progression assessment
To assess technical success and detect post-ablation complications, a contrast-enhanced CT scan was performed immediately after ablation as an ablation confirmation technique. Technical success addresses whether the index tumor was treated according to a predefined protocol and entirely covered by the ablation zone [Citation23]. After 1 month, each patient underwent the first follow-up contrast-enhanced MRI with serum alpha-fetoprotein level. We defined technique efficacy as complete ablation of the index tumor. Patients with complete ablation of the index tumor at the 1-month follow-up imaging were followed up with contrast-enhanced CT or MRI and serum alpha-fetoprotein every 3 months. The primary efficacy rate refers to the percentage of target tumors successfully ablated following the initial ablation, whereas the secondary efficacy rate refers to the percentage of index tumors finally eradicated with repeated ablation. Local control is equivalent to secondary technique efficacy, with the exception of repeated treatments using alternative methods such as transarterial chemoembolization [Citation23]. HCC recurrence patterns after ablation were classified as follows: a) LTP, defined as the appearance of tumor foci at the margin of the ablation zone after the attainment of treatment success; b) intrahepatic remote recurrence, defined as the presence of HCC in the liver at a site discontinuous from the ablation zone; and c) extrahepatic metastasis [Citation24].
Complication assessment
Post-ablation complications were defined as problems noted within 1 month after ablation as well as additional complications identified on follow-up imaging and judged to be likely caused by ablation [Citation23]. Post-ablation complications were graded according to the CIRSE classification system by reviewing medical records and imaging studies [Citation25]. Complications of grade 3 or higher were considered major complications according to the CIRSE classification system, while the rest were considered minor complications.
Statistical analysis
To compare the baseline characteristics, technical success, technique efficacy, and post-ablation complication rates between patients with HCCs in difficult locations and those with HCCs in conventional locations, we used the χ2 or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. We calculated the cumulative incidences of LTP and recurrence-free survival (RFS) using the Kaplan–Meier method, and the differences between the two groups were compared using the log-rank test. RFS was defined as the interval between ablation and the date of any type of recurrence or the last follow-up date if there was no recurrence [Citation23]. Statistical analyses were performed using the MedCalc software (MedCalc version 20.0.23; MedCalc Software, Mariakerke, Belgium).
Results
Technical feasibility rates based on tumor location
Among 456 patients with single HCC ≤ 4 cm, 235 (51.5%) had HCCs in difficult locations: adjacent to large vessels (n = 49), 1st or 2nd branch bile duct (n = 14), diaphragm (n = 100), gallbladder (n = 16), gastrointestinal tract (n = 23), right kidney (n = 10), and pericardium (n = 23) (). Percutaneous ablation was feasible in 431 of 456 (94.5%) patients. Additionally, the feasibility rate was significantly higher in the conventional location group than in the difficult location group (99.5% [220/221] vs. 89.8% [211/235], p < 0.001). The reasons for ablation infeasibility were as follows: a) ≤ 5 mm from the centrally located 1st or 2nd branch bile duct (n = 14); b) lack of safe access route (n = 1); c) exophytic tumor without surrounding liver parenchyma (n = 2); d) failure to secure sonic window (n = 6); and e) failure to ensure adjacent organ protection (n = 2) ().
Figure 2. Percutaneous ablation for a subcardiac hepatocellular carcinoma (HCC) with artificial ascites under fusion image guidance. Gadoxetic acid-enhanced axial (A) and coronal (B) hepatobiliary phase MR images displays a 2.0-cm low signal intensity HCC (arrows) abutting the heart in the left lateral section of the liver. (C) Under fusion imaging guidance, three applicators (arrows) are inserted around the target tumor (red cross marker located at the upper margin of the low echoic target tumor). Artificial ascites (arrowheads) in the perihepatic space not only prevents thermal injury to adjacent organs but also helps insert applicators accurately around the target tumor without puncturing the pericardium. (D) Immediate follow-up contrast-enhanced portal venous phase axial CT demonstrates complete destruction of the target lesion with sufficient ablation margin.

Figure 3. Percutaneous ablation for a subdiaphragmatic hepatocellular carcinoma (HCC) with artificial ascites under fusion image guidance. Contrast-enhanced axial (A) and coronal (B) arterial phase CT images displays a 1.2-cm hypervascular HCC (arrows) abutting the diaphragm in segment VIII of the liver. (C) Fusion imaging technique between real-time working US and reference arterial phase CT images cannot display the target lesion (red cross) due to the lung shadow. (D) After artificial ascites (arrowheads) injection between the diaphragm and liver dome, the target lesion is clearly visualized as a low echoic nodule (red cross) under fusion imaging guidance. (E) After radiofrequency energy delivery, the echo-cloud of micro-bubbles is created around the target lesion. (F) Immediate follow-up contrast-enhanced portal venous phase axial CT demonstrates complete destruction of the target lesion with sufficient ablation margin. (G) There was no local tumor progression at the 32-month follow-up CT.

Figure 4. An infeasible case of percutaneous ablation. Gadoxetic acid-enhanced axial (A) and coronal (B) hepatobiliary phase MR images display a 1.6-cm low signal intensity hepatocellular carcinoma (arrows) abutting the right posterior hepatic duct (arrowheads) in the right liver central portion. Ablation was not performed due to a high risk of central bile duct injury.

The baseline characteristics of the study population are summarized in . The median tumor size in the difficult location group was significantly greater than that in the conventional location group (1.5 cm vs. 1.3 cm, p = 0.003). However, there were no significant differences in the other characteristics between the two groups ().
Table 1. Patients characteristics.
Technical success and post-ablation complications
Among the 431 patients treated with RFA, four patients in the difficult location group and two patients in the conventional location group showed incomplete ablation on immediate CT scans. Among the six patients with incomplete ablation, five patients were treated with repeated ablation, whereas one patient in the conventional location group underwent TACE (technical success, 100% [211/211] vs. 99.5% [219/220], p > 0.999) ().
Regarding post-ablation complications, three patients in the difficult location group and one patient in the conventional location group experienced major complications (grade 3) (1.4% [3/211] vs. 0.5% [1/220], p = 0.363): active bleeding requiring angiographic embolization (n = 2), hemorrhage needing a transfusion (n = 1) in the difficult location group and pleural effusion requiring percutaneous drainage in the conventional location group (n = 1). In addition, there was no significant difference in the rates of all complications between the two groups (6.6% [14/211] vs. 6.4% [14/220], p = 0.909). Detailed information on the major and minor complications is summarized in .
Table 2. Comparison of complication rates between difficult location and conventional location groups.
Technique efficacy and recurrence outcomes: LTP and RFS
In the 1-month follow-up imaging study, three patients in the difficult location group showed viable residual tumors (primary efficacy, 98.6% [208/211] vs. 100% [220/220] in the difficult location and conventional location groups, respectively; p = 0.116). Among patients with residual tumors, one patient was treated with RFA (secondary efficacy, 99.1% [209/211] vs. 100% [220/220]), whereas two patients were treated with TACE (local control rate, 100% [211/211] vs. 100% [220/220]).
During the median follow-up period of 28.9 months (range, 3.7–37.3 months), the estimated 1-, 2-, and 3-year cumulative incidences of LTP of the 211 patients in the difficult location group were 1.0%, 2.5%, and 2.5%, respectively, and were not significantly different from the 2.3%, 3.9%, and 3.9% observed in the 220 patients in the conventional location group (log-rank test, p = 0.456) () (). The estimated 1-, 2-, and 3-year RFS rates were 71.9%, 58.3% and 44.0% and 66.4%, 48.9%, and 36.3% in the difficult location group and the conventional location group, respectively, with no statistically significant difference (log-rank test, p = 0.051) ().
Figure 5. Cumulative incidence estimation of local tumor progression (LTP) and recurrence-free survival (RFS). (A) Cumulative incidence of LTP after ablation in 220 patients in the conventional location group was compared with 211 patients in the difficult location group. (B) Kaplan–Meier estimation of RFS after ablation in 220 patients in the conventional location group was compared with 211 patients in the difficult location group.

Table 3. Comparison of estimated cumulative incidence of local tumor progression between the difficult location and conventional location groups.
Discussion
In our study, among 456 patients with a single HCC ≤ 4 cm, including 235 patients with HCC in difficult locations, percutaneous ablation was feasible in 431 patients (94.5%). While the feasibility rate was significantly different based on tumor locations (89.8% in the difficult location group vs. 99.5% in the conventional location group, respectively), there was no significant difference in the major complication rate between the difficult and conventional location groups (1.4% and 0.5%, respectively). In addition, the estimated 1-, 2-, and 3-year cumulative incidences of LTP in the difficult location group were 1.0%, 2.5%, and 2.5%, respectively, which were not significantly different from the 2.3%, 3.9%, and 3.9% observed in the conventional location group. Our study results are similar to those of previous studies [Citation6,Citation8,Citation10,Citation11] reporting that safe and effective percutaneous ablation can be performed for HCCs in difficult locations. However, previous studies were limited by a small sample size (in the range of 26 to 81 patients), and ablation for HCCs in difficult locations tended to have a high LTP rate compared to conventional tumor locations. In contrast, our study has a strength compared to previous studies in that more than 200 patients were included in each group, and there was no significant difference in the LTP rates between the two groups.
Of note, the overall LTP rate (3.2%) in our study was low in both the conventional and difficult location groups, which is better than the LTP rates reported in previous studies (4.3% to 25.6%) [Citation10,Citation11,Citation26,Citation27]. Several factors contributed to our low LTP rates, including the active use of artificial ascites and/or effusion, a multimodality-US fusion guidance system for improving tumor visibility, real-time monitoring of the ablation zone’s relationship to the index tumor, and the use of multiple applicators and high-power and multichannel generators for large ablation zone creation [Citation14,Citation28]. In addition, we preferentially used more efficient RF energy delivery using either bipolar or dual-switching monopolar modes in the index tumor and peritumoral zone [Citation28–30].
To effectively treat HCCs in the difficult location group, we used several ablation strategies. For tumors in subphrenic locations, artificial ascites between the liver and diaphragm and/or artificial pleural effusion were created to secure a window for index tumor visualization and prevent diaphragm and lung injuries [Citation16,Citation19]. A prior investigation [Citation31] posited that the utilization of artificial ascites during percutaneous ablation could potentially engender a clinical issue through the formation of pleural effusion. However, our study only identified one case where pleural effusion was deemed a clinical problem. For tumors adjacent to the gastrointestinal tract, artificial ascites were injected into the perihepatic space to protect the gastrointestinal tract from thermal injury. Hydrodissection was performed using a guidewire and angiosheath when artificial ascites were not effectively infused due to perihepatic adhesion [Citation12]. Additionally, by continuously injecting artificial ascites while RF energy was delivered, adjacent organs were maximally protected from thermal damage. For tumors in perivascular locations, applicators were inserted parallel to the vessel, and the multiple applicator-RFA approaches using bipolar or switching monopolar mode were applied to overcome the heat sink effect [Citation22,Citation28].
In our study, the major complication rates after ablation were low in both the difficult and conventional location groups (1.4% and 0.5%, respectively). The CT/MRI-US fusion guidance system might have played a crucial role in ensuring the effectiveness and safety of percutaneous ablation for HCCs in our study. Because accurate tumor localization is possible with the imaging fusion technique, the feasibility of ablation could be improved and the number of ablation sessions could be reduced [Citation14,Citation32]. Moreover, since fusion imaging accurately demonstrates the relationship between the ablation zone and adjacent vital organs such as the heart and gastrointestinal tract, it could help minimize collateral thermal injury to adjacent structures. It is possible to precisely evaluate the adequacy of the ablation margin on the real-time working US images referenced by the synchronously moved CT/MR images by accurately identifying the location of the index tumor and echo cloud of microbubbles using the ‘virtual’ target of real-time CT/MR-US fusion imaging technique.
Recent advancements in navigation systems and robotics as well as CT/MR-US fusion imaging techniques have shown promising results in terms of improved treatment outcomes and reduced morbidity after ablation [Citation33,Citation34]. However, further research and development are needed to optimize these technologies and improve their accessibility for a wider range of patients.
Our study has several limitations. First, selection bias may be present due to the fundamental limitations of a retrospective study. However, we attempted to minimize bias by including a large number of patients. Second, this was a single-center study in a tertiary academic hospital where a large volume of ablation was performed. The ablation outcome depends on the operator’s experience; thus, our study results might not be generalizable to other institutions. Third, although there are basic principles for judging ablation feasibility, the final decision was made by the operator. In our study, ablation was performed by one of the four radiologists with various experiences (5–24 years), which might have biased the feasibility rate.
In conclusion, fusion imaging-guided RFA with artificial ascites or pleural effusion could decrease technically infeasible cases, reducing the application of the TSM strategy for small HCCs. Furthermore, it could be an effective treatment for HCCs in difficult locations when feasible, as it provided LTP rates comparable to those of patients with HCCs in conventional locations.
Author contributions
Conceptualization: J.M.L.
Data curation: J.H.K.
Formal analysis: J.H.K.
Funding acquisition: J.M.L.
Investigation: J.M.L., J.Y.L., D.H.L.
Methodology: J.M.L., D.H.L., J.H.Y., I.J., J.H.K.
Project administration: J.M.L.
Resources: J.M.L.
Software: N/A
Supervision: J.M.L., J.Y.L., D.H.L., J.H.Y., I.J., J.Y., Y.J.K., S.J.Y.
Validation: N/A
Visualization: J.H.K.
Writing-original draft: J.M.L., J.H.K.
Writing-review & editing: J.M.L., J.H.Y., I.J., D.H.L., J.H.K., J.Y., Y.J.K., S.J.Y.
Disclosure statement
There are no relevant conflicts of interest related to the submitted work. J.M.L has received grants from Bayer Healthcare, Canon Healthcare, Philips Heathcare, GE Healthcare, CMS, Guerbet, Samsung Medison, and Bracco. J.M.L has received personal fees from Bayer Healthcare, Siemens Healthineer, Samsung Medison, Guerbet, and Philips Healthcare. J.H.Y has received honorarium from Bayer Healthcare and personal fee from Philips Healthcare. For the remaining authors none were declared.
Data availability statement
Raw data were generated at Seoul National University Hospital. Derived data supporting the findings of this study are available from the corresponding author J.M.L. on request.
Additional information
Funding
References
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693.
- European association for the study of the liver. Electronic address eee, european association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60(1):192–201.
- Kwak MH, Lee MW, Ko SE, et al. Laparoscopic radiofrequency ablation versus percutaneous radiofrequency ablation for subphrenic hepatocellular carcinoma. Ultrasonography. 2022;41(3):543–552.
- Mukund A, Ramalingam R, Anandpara KM, et al. Efficacy and safety of percutaneous microwave ablation for hepatocellular carcinomas <4 cm in difficult location. BJR. 2020;93(1116):20191025.
- Xu X-L, Liu X-D, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018;287(2):461–472.
- Filippiadis DK, Spiliopoulos S, Konstantos C, et al. Computed tomography-guided percutaneous microwave ablation of hepatocellular carcinoma in challenging locations: safety and efficacy of high-power microwave platforms. Int J Hyperthermia. 2018;34(6):863–869.
- Ozen M, Birmingham E, Raissi D. Re: liver tumor ablation in difficult locations: microwave ablation of perivascular and subdiaphragmatic hepatocellular carcinoma. Clin Imaging. 2022;85:7.
- Makovich Z, Logemann J, Chen L, et al. Liver tumor ablation in difficult locations: microwave ablation of perivascular and subdiaphragmatic hepatocellular carcinoma. Clin Imaging. 2021;71:170–177.
- Smolock AR, Lubner MG, Ziemlewicz TJ, et al. Microwave ablation of hepatic tumors abutting the diaphragm is safe and effective. AJR Am J Roentgenol. 2015;204(1):197–203.
- Shin SW, Cho SK, Hyun D, et al. Guidewire-catheter induced hydrodissection to assist radiofrequency ablation for subcapsular hepatocellular carcinoma with iodized oil retention in patients with failed artificial ascites due to perihepatic adhesion. Diagn Interv Radiol. 2021;27(6):746–753.
- Cha DI, Kang TW, Song KD, et al. Radiofrequency ablation for subcardiac hepatocellular carcinoma: therapeutic outcomes and risk factors for technical failure. Eur Radiol. 2019;29(5):2706–2715.
- Lee DH, Lee JM. Recent advances in the image-guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, Real-Time multimodality fusion imaging, and new energy sources. Korean J Radiol. 2018;19(4):545–559.
- Bai XM, Cui M, Yang W, et al. The 10-year survival analysis of radiofrequency ablation for solitary hepatocellular carcinoma 5 cm or smaller: primary versus recurrent HCC. Radiology. 2021;300(2):458–469.
- Song I, Rhim H, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19(11):2630–2640.
- Korean Liver Cancer A, National Cancer Center GK Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol 2019. 2018;20:1042–1113.
- Head HW, Dodd GD, 3rd, Dalrymple NC, et al. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology. 2007;243(3):877–884.
- Kang TW, Rhim H, Lee MW, et al. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol. 2011;196(4):907–913.
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61(5):1579–1590.
- Lee DH, Lee MW, Kim PN, et al. Outcome of no-touch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology. 2021;301(1):229–236.
- Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine . 2015;94(9):e580.
- Puijk RS, Ahmed M, Adam A, et al. Consensus guidelines for the definition of time-to-Event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology. 2021;301(3):533–540.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology. 2014;273(1):241–260.
- Filippiadis DK, Binkert C, Pellerin O, et al. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–1146.
- Yang W, Yan K, Wu GX, et al. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: strategies and long-term outcomes. World J Gastroenterol. 2015;21(5):1554–1566.
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(5):317–325.
- Chang W, Lee JM, Lee DH, et al. Comparison of switching bipolar ablation with multiple cooled wet electrodes and switching monopolar ablation with separable clustered electrode in treatment of small hepatocellular carcinoma: a randomized controlled trial. PLOS One. 2018;13(2):e0192173.
- Choi JW, Lee JM, Lee DH, et al. Radiofrequency ablation using a separable clustered electrode for the treatment of hepatocellular carcinomas: a randomized controlled trial of a dual-switching monopolar mode versus a single-switching monopolar mode. Korean J Radiol. 2021;22(2):179–188.
- Choi JW, Lee JM, Lee DH, et al. Switching monopolar radiofrequency ablation using a separable cluster electrode in patients with hepatocellular carcinoma: a prospective study. PLoS One. 2016;11(8):e0161980.
- Song SG, Hur YH, Cho JY, et al. Pleural effusion after hepatic radiofrequency ablation with artificial ascites: clinical spectrum and significance. J Vasc Interv Radiol. 2020;31(10):1636–1644 e1631.
- Ahn SJ, Lee JM, Lee DH, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66(2):347–354.
- Tinguely P, Paolucci I, Ruiter SJS, et al. Stereotactic and robotic minimally invasive thermal ablation of malignant liver tumors: a systematic review and meta-analysis. Front Oncol. 2021;11:713685.
- Schaible J, Pregler B, Verloh N, et al. Improvement of the primary efficacy of microwave ablation of malignant liver tumors by using a robotic navigation system. Radiol Oncol. 2020;54(3):295–300.