Abstract
Introduction
Magnetic Resonance-guided Focused Ultrasound (MRgFUS) thermal ablation is an effective noninvasive ultrasonic therapy to disrupt in vivo porcine tendon but is prone to inducing skin burns. We evaluated the safety profile of a novel hybrid protocol that minimizes thermal spread by combining long-pulse focused ultrasound followed by thermal ablation.
Methods
In-vivo Achilles tendons (hybrid N = 15, thermal ablation alone N = 21) from 15 to 20 kg Yorkshire pigs were randomly assigned to 6 treatment groups in two studies. The first (N = 21) was ablation (600, 900, or 1200 J). The second (N = 15) was hybrid: pulsed FUS (13.5 MPa peak negative pressure) followed by ablation (600, 900, or 1200 J). Measurements of ankle range of motion, tendon temperature, thermal dose (240 CEM43), and assessment of skin burn were performed in both groups.
Results
Rupture was comparable between the two protocols: 1/5 (20%), 5/5 (100%) and 5/5 (100%) for hybrid protocol, compared to 2/7 (29%), 6/7 (86%) and 7/7 (100%) for the ablation-only protocol with energies of 600, 900, and 1200 J, respectively. The hybrid protocol produced lower maximum temperatures, smaller areas of thermal dose, fewer thermal injuries to the skin, and fewer full-thickness skin burns. The standard deviation for the area of thermal injury was also smaller for the hybrid protocol, suggesting greater predictability.
Conclusion
This study demonstrated a hybrid MRgFUS protocol combining long-pulse FUS followed by thermal ablation to be noninferior and safer than an ablation-only protocol for extracorporeal in-vivo tendon rupture for future clinical application for noninvasive release of contracted tendon.
Introduction
Contractures are the shortening of the muscle-tendon unit causing weakness and decreased range of motion (ROM) about a joint, which often leads to deformity, disuse, pain, and disability [Citation1–3]. In severe cases, non-surgical interventions such as physiotherapy and splints are unable to achieve long-term improvements in ROM. Surgical tendon resection is the most effective treatment for contractures; however, it is associated with recurrence, anesthetic and surgical risk. Furthermore, not all patients are suitable surgical candidates [Citation4]. Children with cerebral palsy are particularly prone to tendon contracture, and may undergo multiple significant surgical procedures, at multiple joint levels with prolonged rehabilitation, over their lifetime. All age groups however are vulnerable to joint contracture from accident, congenital malformation, and stroke being amongst the most common.
High intensity focused ultrasound (HIFU) uses extracorporeally placed transducers to focus ultrasound beams within the body [Citation5–7]. When used alongside Magnetic Resonance Imaging (MRI) guidance for treatment planning and monitoring, the therapy is referred to as Magnetic Resonance-guided Focused Ultrasound (MRgFUS). MRgFUS has numerous clinical applications and has potential to reduce the pain of open surgical procedures, shorten hospital stay, allow graded or multiple procedures to fine tune the tendon release, reduce post-operative recovery time and minimize side effects such as ionizing radiation to minimize future malignancy in the pediatric population [Citation8,Citation9]. Ultrasound parameters can be modulated to induce both thermal or mechanical bio-effects in vivo [Citation10].
During thermal ablation procedures, heating is achieved by energy absorption at the focal point. Irreversible tissue damage occurs when tissue heating achieves a thermal dose of 240 cumulative equivalent minutes at 43 °C (240 CEM43) [Citation5,Citation11–13]. Tendon ablation is efficient due to its high acoustic attenuation coefficient of 2.9 dB/(MHz cm) [Citation14]. However, unintended thermal spread from the focus or skin bubbles can cause skin thermal injuries for this superficial target. This has been observed despite the use of real-time tissue temperature monitoring using proton resonance frequency shift MR thermometry (PRFS-MRT), which produces quantitative temperature maps with an uncertainty < 1 °C [Citation15–18].
Long-Pulse Focused Ultrasound is a mechanically induced ultrasound bioeffect in tissue achieved by exciting the transducer with low duty cycles to create pulses of high amplitude [Citation19] Millisecond-long pulses at higher frequencies (1–3 MHz) lead to the formation of shockwaves due to non-linear propagation effects of the acoustic waveform [Citation20,Citation21]. High frequency shockwaves cause rapid and precise tissue heating that is 20 times more efficient than heating caused by linear acoustic waves [Citation20]. Because of the transient nature of the heating, thermal diffusion is negligible [Citation21]. This localized heating forms a boiling bubble at the focal point which grows to form a millimeter-sized vapor cavity [Citation20]. The interaction of subsequent shock waves with this cavity results in mechanical fractionation of tissue accompanied with little to no thermal effects other than at the microscopic boiling point [Citation20].
Previous in vivo porcine experiments found that MRgFUS thermal ablation can be used to effectively rupture Achilles tendons and improve ROM [Citation17]. A complication of these therapies was thermal spread around the focal point leading to large areas of thermal injury at the skin surface, limiting its applicability in patients. The primary objective of this study is to evaluate pulsed FUS as a pre-ablation step to seed boiling bubble clouds that remain in situ and effectively disrupt tendons while minimizing thermal spread when compared to an ablation only treatment. Secondary objectives include assessment of temperature distribution, thermal dose margins, thermal injury to overlying skin, change in range of motion, and histological characterization of the treatment, which will be compared to previous experiments of ablation only treatment.
Methodology
Animal Preparation: All animal procedures were approved by the Animal Care Committee at the Hospital for Sick Children (Toronto, Ontario, Canada). Achilles tendons (n = 36) from healthy 15–20 kg Yorkshire pigs were chosen due to the animal’s anatomic and physiologic similarities to human tendon properties [Citation22]. Animals received Acepromazine/Atropine/ketamine (0.1/5/11.9 mg/kg) (Ketalean, CDMV Quebec, Canada) as a preanesthetic, and were then intubated and ventilated with inhalant anesthesia (2.5% isoflurane in 2 L oxygen). Maintenance fluids (0.9% saline with dextrose) were delivered using a 22 G angiocath during the experiment. Body temperature was monitored through a rectal probe and maintained at approximately 37 °C using a circulating water heating blanket. Vital signs (SP02, and ETCO2) were monitored using an MR-compatible system.
Goniometry
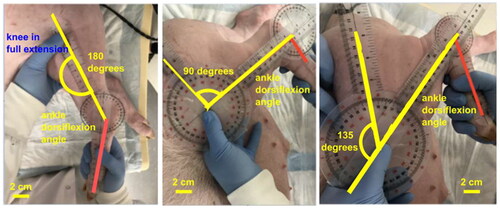
For each limb, the range of motion (ROM) of the ankle joint was determined for the Achilles tendon, gastrocnemius and soleus muscle complex. To account for the tightness of the gastrocnemius, maximal dorsiflexion was measured with the knee fixed in three positions: fully extended (0° flexion), 90° flexion, and 135° flexion as shown in . Maximal dorsiflexion of the ankle joint was assessed with a goniometer by a senior orthopedic surgery resident with two non-clinical lab members arriving at a consensus maximal angle of measurement.
MRgFUS setup, planning, and treatment
The animal was positioned as reported previously, laterally feet first on the MRgFUS table with the Achille’s tendon centered within the MRI bore [Citation17]. Acoustic coupling was achieved by layering degassed reversed osmosis water-gel mixture with a 3.5 mm ultrasound gel pad (Aquaflex, Parker Laboratories, Fairfield, NJ, USA). A 3 T Achieva MRI system (Philips Healthcare, Best, the Netherlands) and a Sonalleve three channel pelvic coil were used to acquire planning images, verify absence of air bubbles, and perform real-time thermometry with proton resonance frequency shift MR thermometry (PRFS-MRT). The PRFS-MRT sequence has a temperature uncertainty of less than 1 °C and a spatial accuracy determined by the imaging resolution of 1.5 × 1.5 × 5.5 mm [Citation16,Citation23–25]. This sequence is FDA-approved for clinical use in soft tissues and has been validated by other groups [Citation16,Citation26]. The coupling scan is a T1-w Fast Field Echo (FFE) sequence that was performed to check for small air bubbles trapped between the gel pad and skin which can lead to near-field skin thermal injuries, and was performed repeatedly until at least two investigators agreed upon optimal coupling. The MRI pulse sequences are summarized in .
Table 1. Summary of MRI and PRF-based MRT pulse sequence parameters.
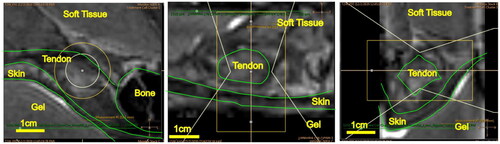
MRgFUS treatment was delivered using a clinically-approved Sonalleve V1 MR-HIFU system (Profound Medical Inc., Ontario, Canada) with transducer frequency = 1.2 MHz, number of elements = 256, surface diameter = 128 mm, and radius of curvature = 120 mm at single focused focal spot size of 1.5 × 1.5 × 9.2 mm (assuming linear wave propagation) when a 2 mm diameter treatment focal point is employed, as reported in the literature [Citation27]. Each Achilles tendon received two adjacent treatments within 1 mm to achieve sufficient coverage of the tendon. Following the first treatment, a new T2 weighted MR image was acquired to confirm accurate targeting, and was used to plan the second sonication point. An example of this step in the procedure is provided in .
Figure 2. T2-weighted MRI image of the Achilles tendon used in treatment planning of MRgFUS sonication. Treatment target is verified in the (a) sagittal, (b) axial, and (c) coronal views.
Two protocols were compared in this study. In a previous study, the ablation protocol (A) delivered a continuous wave sonication at 1.2 MHz for 30s at acoustic powers of 20 W (n = 7), 30 W (n = 7), or 40 W (n = 7). Each respective power applied for 30 s is equivalent to 600 J, 900 J, 1200 J as reported to Chu Kwan, et al. [Citation17]. These ablation treatments are labeled Group 1, 2, and 3 A, respectively. In this study, we conducted a hybrid protocol (B) consisted of a pulsed FUS sonication for 60 s followed by a thermal ablation sonication at acoustic powers of 20 (n = 5), 30 (n = 5) or 40 (n = 5) Watts for 30 s. These hybrid treatments are labeled Group 1B, 2B, and 3B, respectively. For pulsed FUS, a peak negative pressure of 13.5 MPa was applied at 1.2 MHz with a duty cycle of 1%, and a pulse duration of 0.01 s for 12,000 pulses per burst base on previous characterization which corresponds to a peak positive pressure of 35 MPa as reported by Karzova et.al [Citation27,Citation28]. Calibration of the pulse FUS was done with a fiberoptic hydrophone in a water tank. The ablation sonication was delivered within 15 s of the end of the pulsed FUS sonication. Continuous temperature monitoring was performed with a dynamic scan time of 1.7s using PRFS-MRT with a zero-order drift correction to assess the heating of tissues on and off target with spatial accuracy of 1.5 mm [Citation29]. Temperature monitoring was performed continuously for 5 min after the end of the ablation sonication to ensure return of temperature to physiologic baseline. All ablation experiments were performed prior to all hybrid experiments as part of a previously published study [Citation17]. Treatment groups were randomized and assigned on the day of experimentation.
Post-treatment goniometry and necropsy
T2-weighted images of the tendons were acquired following treatment. The ankle was then forcefully dorsiflexed by a senior orthopedic surgery resident to the maximum ROM to disrupt the tendon. Goniometry was repeated as described above. Animals were euthanized with sodium pentobarbital (120 mg/kg) within 15 min followed by necropsy and gross anatomy examination. Histology preparation involved fixation in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin for analysis of tendon and skin tissue. Once histology was processed, microscopic examination of cross-sectioned tendons was under taken.
Treatment temperature analysis
The maximum temperature of a treatment was defined as the average temperature within a 3 × 3 voxel region surrounding the highest temperature pixel following the thermal ablation sonication. No thermometry analysis was performed during pulsed FUS. Analysis was performed by two authors blinded to the treatment protocol, and conducted at three timepoints of interest: immediately after treatment, 1 min after treatment, and 5 min after treatment to verify return to physiologic baseline (37 °C). Homogeneity of variance tests (Levene’s) and normality tests (Shapiro-Wilk) were used to determine whether to proceed with parametric or non-parametric statistical assessment. Tukey and Wilcoxon tests were used to compare maximum temperatures between treatment protocols from each energy group.
Thermal lesion analysis
A thermal dose measurement was performed to estimate the area of tissue that was met the threshold for necrosis by the therapy. The Sonalleve system computes and displays the boundary that surpasses the threshold of the 240 cumulative equivalent minutes at 43 °C (240 CEM43), which predicts a 100% lesion probability [Citation11,Citation12]. The area within the 240 CEM43 region in the coronal plane was manually measured using ImageJ by two blinded authors [Citation30]. Analysis was performed for both the hybrid and ablation-only protocols immediately following the ablation treatment and 240 CEM43 areas between these protocols was compared using the Wilcoxon non-parametric test.
Skin injury analysis
The primary endpoint for this study was tendon disruption, which was subsequently confirmed after gross anatomy and necropsy analysis. Fisher’s exact test was used to compare rupture frequency between the treatment protocols. The average focal depth for each treatment protocol was calculated by averaging the distance from the sonication point to the near-field surface, the far-field surface, and the posterior skin surface. Skin lesions were photographed and the area was measured using ImageJ by two blinded authors [Citation30]. Normality tests was verified with the Shapiro–Wilk test, followed by ANOVA to compare the focal depth and size of thermal injury treatment protocols in each energy group.
Results
Tendon release and goniometry results
The percentage of tendons ruptured in each treatment group is summarized in . Tendon rupture for the hybrid protocol was 1/5 (20%), 5/5 (100%) and 5/5 (100%) for the 600, 900, and 1200 J groups respectively. Tendon rupture for the ablation-only protocol was 2/7 (29%), 6/7 (86%) and 7/7 (100%) for the 600, 900, and 1200 J groups respectively. Fisher’s exact test was used to compare the frequency of rupture for Protocol A versus Protocol B and the rupture frequency was not statistically different (p = 0.2691).
Table 2. Summary of treatment groups designation and results of tendons ruptured and goniometry range of motion change for each treatment group.
The changes in ROM as measured by goniometry are shown in (Supplemental Figure 1). For example, with the knee fixed at 90°, the gain the angle of the ankle at maximum dorsiflexion was 12 ± 9, 16 ± 7 and 27 ± 11 degrees for groups 1, 2 and 3 A, and 5 ± 3, 12 ± 8 and 33 ± 7 degrees for 1B, 2B and 3B respectively. Protocols A and B were compared using ANOVA and gains in ROM were also not significantly different between the two protocols.
Treatment Temperature Analysis: The maximum temperatures at the focal point immediately following the hybrid protocol were 46.52 ± 2.72, 55.07 ± 4.85 and 57.12 ± 5.36 degrees for the 600, 900, and 1200 J groups respectively. The maximum temperatures immediately following the ablation-only therapy were 58.38 ± 2.70, 63.34 ± 3.20 and 67.58 ± 4.67 degrees for the 600, 900, and 1200 J groups respectively. (Supplemental Figure 2) summarize the maximum temperatures for each treatment group at 3 timepoints (immediately, 1 min, and 5 min after treatment). and provide an example showing the maximum temperatures achieved for Group 2B (pulsed FUS and 900 J) and 2 A (900 J alone), where lower temperatures were observed in Group 2B. Statistical analysis demonstrated that all the maximum temperatures were significantly lower for the hybrid protocol when compared to the ablation-only protocol for each energy dose (p < 0.001), except at 5 min, which was expected as the tissue has returned to physiologic baseline in both cases.
Figure 3. T2-weighted MRI image of the Achilles tendon with MR thermometry (PRFS-MRT) at the end of the MRgFUS thermal ablation sonication showing the maximum temperature achieved for Group 2B (pulsed FUS and 900 J). Temperature mapping corresponds to the scale (blue = 40°, yellow = 55°, red = 70°) displayed on the right for PRFS-MRT in (a) sagittal, (b) axial, and (c) coronal views. The white lines represent the sonication cone; the yellow lines represent the treatable sonication region.
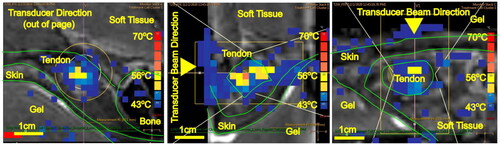
Figure 4. MRI image of the Achilles tendon with MR thermometry (PRFS-MRT) at the end of MRgFUS sonication showing the maximum temperature achieved for Group 2 A (900 J only). Temperature mapping corresponds to the scale displayed on the right for PRFS-MRT in (a) sagittal, (b) axial, and (c) coronal views. The white lines represent the sonication cone; the yellow lines represent the treatable sonication region.
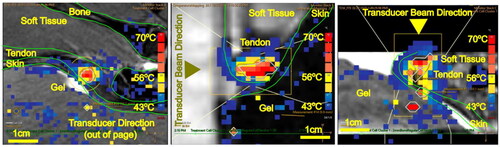
Table 3. Summary of maximum temperatures for each treatment group at three timepoints.
Thermal lesion results
The thermal lesion areas for computed 240 CEM43, through the cross section of the focal point, were 20.80 ± 15.52, 36.66 ± 16.25 and 43.18 ± 30.40 mm2 for Groups 1, 2 and 3 A, and 6.00 ± 6.32, 20.4 ± 9.88 and 36 ± 14.96 mm2 for Groups 1B, 2B and 3B respectively, as summarized in (Supplemental Figure 3). The 240 CEM43 areas are significantly smaller between the hybrid and the ablation protocols at 600 and 900 J (p < 0.05), but not at 1200 J.
Skin Thermal Injury analysis: The average distance from the sonication point to the skin surface did not exhibit statistically significant disparities across the respective groups, thereby establishing a reliable basis for comparing the incidence of skin burns (). The average skin thermal injury areas are plotted in (Supplemental Figure 4) for each treatment protocol. For the ablation protocol, the injury areas averaged 5.4 ± 7.1, 21.8 ± 289 and 37.1 ± 35.6 mm2 for 1, 2 and 3 A. For the hybrid protocol the areas for 1B, 2B and 3B averaged 5.3 ± 2.6, 9.8 ± 11.7 and 23.5 ± 9.1 mm2 respectively. ANOVA and Tukey’s test showed no statistical differences in skin injury area between the hybrid and ablation protocols. The prevalence of skin burns was 76% (16/21) in the ablation protocol and 66% (10/15) in the hybrid protocol. The prevalence of full-thickness skin was more in the ablation protocol (9/21 = 43%) compared to for the hybrid protocol (1/15 = 6%), which only occurred in the 1200 J group.
Table 4. Summary of skin necrosis for each treatment group.
Discussion
This study is the first study to describe a hybrid protocol combining MRI-guided long- pulsed FUS followed by thermal ablation with the goal to noninvasively disrupt tendons. There was comparable effectiveness for tendon disruption for the hybrid protocol and the ablation alone protocol. The change in range of motion between protocols was not statistically significant different which can be attributed to the wide standard deviation of the ankle ROM when the knee was at 135°. This could be attributed to human measurement error as it is difficult to achieved a flexion of 135° given the swine soft tissue envelope. Another aim of this study was to determine whether preceding ablation with pulsed FUS could result more predictable tissue heating with reduced thermal spread improving the safety of the procedure. When compared to the ablation only protocol, we observed lower maximum temperature for the hybrid protocol, which is desirable to reduce thermal spread and undesired damage to adjacent structures such as muscles, nerves, and skin. These lower temperatures also translated into a reduced extent of thermal spread - as indicated by the area of the computed 240 CEM43 boundary – being statistically significantly smaller. This is likely due to the larger standard deviation in the 240 CEM43 areas at high energy doses for the ablation protocol. Care was taken to ensure that baseline temperatures of all animals and environment temperatures were comparable between experiments.
In any thermal therapy, collateral and unnecessary heating of surrounding healthy tissue is undesirable from a safety standpoint. Although there was no statistically significant difference in the area of skin thermal injury, the standard deviations of the skin burn area from the hybrid protocol were narrower, which is indicative of greater predictability. There were a fewer number of skin thermal injuries and less full thickness skin injuries using the hybrid protocol. Temperature and thermal spread analysis indicate that the hybrid treatment approach may be a safer option than using ablation alone. This improvement would provide a more predictable and safer protocol to reduce skin burns, especially when coupled with burn mitigation strategies such as temperature monitoring and active cooling. While most burns were classified as minor, there were a few that included regions of thermal necrosis. Villapos et al. presented a case of a full thickness abdominal burn with necrosis following an MRgFUS treatment of uterine fibroids [Citation31]. While initially treated with antibacterial cream due to a region of hyperintensity on the overlaying skin, the patient was referred to a burn unit two weeks following the treatment. There was evident damage to the abdominal fascia and necrosed tissue was excised, requiring an overnight hospital stay [Citation31]. Based on this case, it is possible that the nature of the burns seen on the animals may have changed had a longer-term study been possible.
In an ideal clinical therapy, there would be no skin burns resulting from the treatment. This could be achieved by aborting the sonication when real-time temperature monitoring shows significant heating of the skin. Because this was a feasibility study, treatments were not aborted despite sometimes being able to visualize thermal spread. Most skin burns caused by the treatment were in the near field, and there are some straightforward yet effective steps that can be taken to reduce this risk. Active cooling pads and customized gel pads made out of a rigid material such as agar that the limb could sink into could improve skin-gel coupling. Real-time monitoring with high resolution images will be critical to ensure safe treatments, and abort treatment if there is concern of collateral damage. This study demonstrates feasibility of a hybrid long-pulse FUS protocol followed by thermal ablation to be statistically similar to previous work which used ablation alone with a safer temperature and a smaller area of the 240 CEM43 boundary, narrower standard deviation in area skin thermal injury, and fewer number of full-thickness skin injuries.
Despite the promising findings of this study, quantitatively measuring the peak positive pressure will provide confirmation of achieved either long-pulse histotripsy and boiling histotripsy [Citation32,Citation31], providing an objective measurement of pre-ablation treatment. For histology, thermal injury histology stain such as NADH-d will be used for future studies. This histology will further confirm and depict the extend of thermal injury in the tendon and in surrounding tissue. Additionally, ROM measurement can be further improved with objective measurement of the knee ROM (full extension, 90°, 135°) using X-ray imaging to confirm the goniometry measurement. Finally, these studies were performed in normal tendons while the ultimate goal is the noninvasive treatment of contracted tendons. Future studies will focus on treatment of a tendon contracture model. All these study design considerations will be included in future studies.
Conclusion
This paper presents a proof-of-concept study that compares the efficacy and safety of a continuous FUS protocol against a hybrid long-pulse FUS followed by continuous FUS for the noninvasive disruption of tendons. We have demonstrated that the hybrid protocol provides the same rate of tendon disruption with a more predictable safety profile for skin burns. This suggests potential future clinical use for noninvasively releasing contracted tendons.
Supplemental Material
Download MS Word (307.5 KB)Acknowledgements
We would like to acknowledge Dr. Unni G. Narayanan for his clinical input, Mr. Bryan Maguire for his consultation on the data analysis, and Marvin Estrada and Anson Lam for their support in animal husbandry.
Disclosure statement
Ari Partanen is an employee of Profound Medical, Mississauga, Canada. The remaining authors report there are no competing interests to declare.
Data availability statement
Raw data were generated at the Posluns Center for Image Guided Innovation and Therapeutic Intervention, The Hospital for Sick Children. Derived data supporting the findings of this study are available from the corresponding author WCK on request.
Additional information
Funding
References
- Fergusson D, Hutton B, Drodge A. The epidemiology of major joint contractures: a systematic review of the literature. Clin Orthop Relat Res. 2007;456:1–7. doi:10.1097/BLO.0b013e3180308456.
- Bartoszek G, Fischer U, Müller M, et al. Outcome measures in older persons with acquired joint contractures: a systematic review and content analysis using the ICF (International Classification of Functioning, Disability and Health) as a reference. BMC Geriatr. 2016;16(1):40. doi:10.1186/s12877-016-0213-6.
- Rabiner A, Roach KE, Spielholz NI, et al. Characteristics of nursing home residents with contractures. Phys Occup Ther Geriatr. 1996;13(4):1–10. doi:10.1080/J148v13n04_01.
- Farmer SE, James M. Contractures in orthopaedic and neurological conditions: a review of causes and treatment. Disabil Rehabil. 2001;23(13):549–558. doi:10.1080/09638280010029930.
- van Rhoon GC, Samaras T, Yarmolenko PS, et al. CEM43 °C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol. 2013;23(8):2215–2227. doi:10.1007/s00330-013-2825-y.
- Shaw CJ, ter Haar GR, Rivens IH, et al. Pathophysiological mechanisms of high-intensity focused ultrasound-mediated vascular occlusion and relevance to non-invasive fetal surgery. J R Soc Interface. 2014;11(95):20140029. doi:10.1098/rsif.2014.0029.
- ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104. doi:10.1080/02656730601186138.
- Tempany CMC, McDannold NJ, Hynynen K, et al. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259(1):39–56. doi:10.1148/radiol.11100155.
- Dorfman AL, Fazel R, Einstein AJ, et al. Use of medical imaging procedures with ionizing radiation in children: a population-based study. Arch Pediatr Adolesc Med. 2011;165(5):458–464. doi:10.1001/archpediatrics.2010.270.
- Miller DL, Smith NB, Bailey MR, et al. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31(4):623–634. doi:10.7863/jum.2012.31.4.623.
- McDannold N, Vykhodtseva N, Jolesz FA, et al. MRI investigation of the threshold for thermally induced blood–brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med. 2004;51(5):913–923. doi:10.1002/mrm.20060.
- McDannold N, Livingstone M, Top CB, et al. Preclinical evaluation of a low-frequency transcranial MRI-guided focused ultrasound system in a primate model. Phys Med Biol. 2016;61(21):7664–7687. doi:10.1088/0031-9155/61/21/7664.
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800. doi:10.1016/0360-3016(84)90379-1.
- Nassiri DK, Nicholas D, Hill CR. Attenuation of ultrasound in skeletal muscle. Ultrasonics. 1979;17(5):230–232. doi:10.1016/0041-624x(79)90054-4.
- Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34(6):814–823. doi:10.1002/mrm.1910340606.
- Mougenot C, Köhler MO, Enholm J, et al. Quantification of near-field heating during volumetric MR-HIFU ablation. Med Phys. 2011;38(1):272–282. doi:10.1118/1.3518083.
- Chu Kwan W, den Otter-Moore I, Partanen A, et al. Noninvasive magnetic resonance-guided focused ultrasound for tendon disruption: an in vivo animal study. Int J Hyperthermia. 2023;40(1):2260129. doi:10.1080/02656736.2023.2260129.
- Muratore R, Akabas T, Muratore IB. High-Intensity focused ultrasound ablation of ex vivo bovine achilles tendon. Ultrasound Med Biol. 2008;34(12):2043–2050. doi:10.1016/j.ultrasmedbio.2008.05.006.
- Canney MS, Khokhlova VA, Bessonova OV, et al. Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound Med Biol. 2010;36(2):250–267. doi:10.1016/j.ultrasmedbio.2009.09.010.
- Maxwell A, Sapozhnikov O, Bailey M, et al. Disintegration of tissue using high intensity focused ultrasound: two approaches that utilize shock waves. Acou Today. 2012;8(4):24. doi:10.1121/1.4788649.
- Khokhlova VA, Fowlkes JB, Roberts WW, et al. Histotripsy methods in mechanical disintegration of tissue: toward clinical applications. Int J Hyperthermia. 2015;31(2):145–162. doi:10.3109/02656736.2015.1007538.
- Zhang G, Zhou X, Hu S, et al. Large animal models for the study of tendinopathy. Front Cell Dev Biol. 2022;10:1031638. doi:10.3389/fcell.2022.1031638.
- Mougenot C, Quesson B, De Senneville BD, et al. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU). Magn Reson Med. 2009;61(3):603–614. doi:10.1002/mrm.21887.
- Köhler MO, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36(8):3521–3535. doi:10.1118/1.3152112.
- Senneville BD, Mougenot C, Quesson B, et al. MR thermometry for monitoring tumor ablation. Eur Radiol. 2007;17(9):2401–2410. doi:10.1007/s00330-007-0646-6.
- Humanitarian Device Exemption (HDE). Accessed July 30, 2023. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=H190003.
- Karzova MM, Kreider W, Partanen A, et al. Comparative characterization of nonlinear ultrasound fields generated by sonalleve V1 and V2 MR-HIFU systems. IEEE Trans Ultrason Ferroelectr Freq Control. 2023;70(6):521–537. doi:10.1109/TUFFC.2023.3261420.
- Raghuram H, Looi T, Pichardo S, et al. A robotic MR-guided high-intensity focused ultrasound platform for intraventricular hemorrhage: assessment of clot lysis efficacy in a brain phantom. J Neurosurg Pediatr. 2022;30(6):586–594. doi:10.3171/2022.8.PEDS22144.
- Bing C, Staruch RM, Tillander M, et al. Drift correction for accurate PRF-shift MR thermometry during mild hyperthermia treatments with MR-HIFU. Int J Hyperthermia. 2016;32(6):673–687. doi:10.1080/02656736.2016.1179799.
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi:10.1038/nmeth.2089.
- Leon-Villapalos J, Kaniorou-Larai M, Dziewulski P. Full thickness abdominal burn following magnetic resonance guided focused ultrasound therapy. Burns. 2005;31(8):1054–1055. doi:10.1016/J.BURNS.2005.04.019.
- Wang YN, Khokhlova T, Bailey M, et al. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound Med Biol. 2013;39(3):424–438. doi:10.1016/j.ultrasmedbio.2012.10.012.
- Khokhlova TD, Canney MS, Khokhlova VA, et al. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J Acoust Soc Am. 2011;130(5):3498–3510. doi:10.1121/1.3626152.