Abstract
Background
Swedish recommendations to reduce the risk of COVID-19 relied on each citizen’s own sense of responsibility rather than mandatory lockdowns. We studied how COVID-19-related self-isolation and anxiety correlated to SARS-CoV-2 seropositivity and PCR-positivity in patients with active cancer treatment.
Methods
In a longitudinal cohort study at Uppsala University Hospital patients and cancer personnel were included between April 1st 2020 to August 1st 2020. Serological testing for SARS-CoV-2 was done every 8–12-weeks until 30 March 2021. Patients completed a survey at inclusion regarding self-reported COVID-19-related anxiety and self-isolation.
Results
A total of 622 patients [n = 475 with solid malignancies (SM), n = 147 with haematological malignancies (HM)], and 358 healthcare personnel were included. The seropositivity rate was lower for patients than for personnel; 10.5% for SM patients, 6.8% for HM patients, and 16.2% for personnel (p = 0.005). Strict adherence to self-isolation guidelines was reported by 54% of patients but was not associated with a lower risk of becoming seropositive [OR = 1.4 (0.8–2.5), p = 0.2]. High anxiety was expressed by 32% of patients, more often by SM patients than HM patients (34% vs 25% [OR = 1.6 (1.1–2.5, p = 0.03)]). Female gender [OR = 3.5 (2.4–5.2), p < 0.001] and being born outside of Europe [OR = 2.9 (1.4–6.4), p = 0.007] were both associated with high anxiety. Patients reporting high anxiety became seropositive to a similar degree as those with low anxiety [OR = 0.7 (0.3–1.2), p = 0.2]. HM patients with PCR-positive COVID-19 were more likely than SM patients to require oxygen therapy, including non-invasive ventilation/intubation (69% vs. 26%, p = 0.005).
Conclusion
For Swedish patients on active cancer treatment, high self-assessed COVID-19-related anxiety or strict adherence to self-isolation guidelines were not associated with a lower risk of COVID-19. Patients with HM were less likely to develop serological antibody response after COVID-19 and were more likely to require advanced hospital care, but expressed less COVID-19-related anxiety than patients with SM.
Introduction
During the early phases of the COVID-19 pandemic in 2020 many countries declared mandatory lockdowns or curfews and closed primary and secondary schools in order to reduce the spread of the disease and to alleviate pressures on healthcare systems [Citation1]. In Sweden, however, a different strategy was adopted; avoiding mandatory lockdowns and opting to keep schools and workplaces open, while encouraging individuals to voluntarily work from home whenever possible. This approach relied heavily on the individual’s own sense of responsibility for adherence to guidelines and social distancing measures.
Cancer disease was early on identified as a possible risk factor for COVID-19, including an increased risk of severe disease and death [Citation2,Citation3], at a time when there was no proven effective COVID-19 treatment or vaccine yet available [Citation4,Citation5]. Cancer patients are usually extensively informed regarding the increased risk of infections during cancer treatments. Adequate COVID-19-specific protective measures for cancer patients were not established at the start of the pandemic, possibly increasing anxiety in patients and affecting attitudes both towards discontinuing cancer treatment for fear of infection [Citation6] and towards the import of protective measures such as self-isolation. Delaying cancer treatment out of fear of SARS-CoV-2 exposure could result in adverse outcomes and progressive disease while suffering a SARS-CoV-2 infection during treatment might negatively affect the results of planned treatments. It was therefore of interest to study the effects of COVID-19 on cancer patients, especially in the Swedish setting of no mandatory lockdowns and no requirement to wear masks.
Our aim was to examine the cumulative PCR- and seropositivity for SARS-CoV-2, and the possible association with patient characteristics, self-reported COVID-19-related anxiety and self-isolation, as well as disease severity and mortality, in actively treated cancer patients during the first year of the COVID-19 pandemic until the advent of vaccination programs.
Material and methods
Study design and inclusion
A longitudinal prospective cohort study was conducted at Uppsala University Hospital (Akademiska Sjukhuset, Uppsala) in Sweden, between 1 April 2020 until 30 March 2021. Eligible patients for the study were identified using planned appointments for any form of active antitumoral treatment (chemotherapy, monoclonal antibodies, tyrosine kinase inhibitors, immunotherapy – BCL2-inhibitors, PD1/PDL1-inhibitors, CDK4/6-inhibitors, BRAF/MEK-inhibitors) found through electronic medical records. Patients undergoing active treatment between 1 April 2020 and 1 August 2020 were offered study participation either by telephone or at in-house appointments by study investigators or personnel in charge of their care, and positive responders were thereafter included in the study by the study investigators. Personnel at the Department of Oncology and Hematology received information regarding the study via e-mail and participation was voluntary.
Follow-up
Since vaccination can result in a positive serological test, seropositivity was investigated in this study up until general vaccination programs commenced. Patients were followed until (1) death, (2) active antitumoral treatment was considered completed and patients had no additional planned health care visits, or (3) initiation of vaccination against SARS-CoV-2. Personnel was followed until vaccination was initiated. In general, patients in our care received their first vaccination between February and May 2021 and personnel from January 2021 onwards.
Controls
Healthcare personnel were chosen as a control group due to a) being considered immunocompetent compared to patients with cancer and b) having a pre-existing reason to go to the hospital for their work. This would allow serological testing of healthy controls without the potential of additional viral exposure to individuals from the general population, i.e., healthy and elderly individuals without cancer who lacked any other reason to visit healthcare facilities, thereby reducing community exposure in line with current guidelines.
Masks were not mandatory in Sweden at any time point except for health care personnel who were required to wear them in duty. The effect of this policy was not investigated in this study.
Ethical approval
The study was ethically approved according to the Declaration of Helsinki from the Swedish Ethical Review Authority (Dnr: 2020-01781 and Dnr: 2020-03195). All study participants (patients and staff) provided signed written informed consent at study inclusion and before collection of the first blood sample.
Patient population
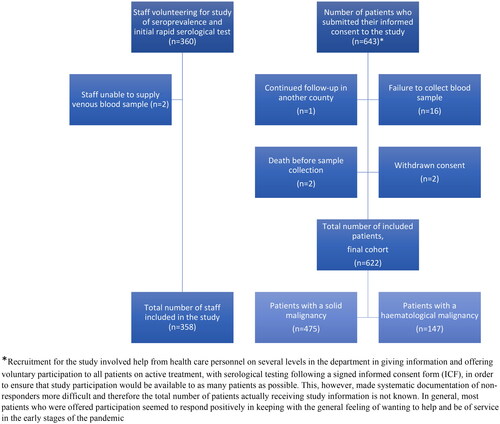
In total, n = 643 individuals with active anti-cancer treatment were included in the study, n = 21 patients were excluded from the study due to failure in sample collection, withdrawn consent, death before first sample collection or follow-up continued in another county ().
Health-care personnel
Of the 360 included personnel, out of about 420 full and part-time employees, 358 (86%) provided at least one sample for serological testing (n = 2 were unable to supply blood samples at the start of the study).
Survey on anxiety and attitudes towards self-isolation
Patients, but not personnel, were asked to complete a survey regarding anxiety related to the COVID-19 pandemic, adherence to self-isolation and social distancing guidelines, birthplace, their current living situation and the presence of minors in the household (Supplementary material). The survey was completed once, at inclusion (April 1st to August 1st 2020). The level of self-assessed COVID-19-related anxiety was divided into four categories; none, mild, moderate, or high. Adherence to guidelines regarding social distancing and self-isolation was divided into three categories (not at all, partly or completely). For the purpose of statistical analysis the degree of anxiety was divided into two categories (none–mild and moderate–high) and the degree of self-isolation was further divided into a comparison of those that (1) did not at all or only partly follow guidelines versus (2) those that fully followed guidelines.
Collection of data, serological and PCR-testing in patients and personnel
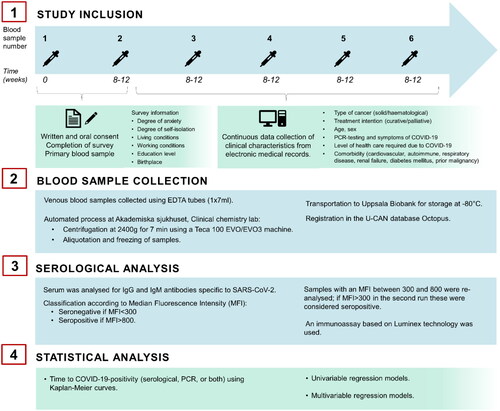
Clinical data concerning patients’ diagnosis, staging, treatment intention, and oncologic therapy were collected from electronic medical records. For patients, follow-up within the study initially entailed the collection of blood samples every 6 to 8 weeks, which was gradually extended to every 12 weeks, throughout the next 12 months of the pandemic, estimating a minimum of 4–6 samples per patient (). Blood samples were collected according to each patient’s individual health care plan during routine visits or pre-planned in-patient care, to ensure that the study itself would not contribute to additional community exposure and risk of contracting a SARS-CoV-2 infection. The results of nose swabs for PCR-testing for SARS-CoV-2 were gathered from medical records, including the reason for conducting this test, whether it be as screening for all hospitalized patients or due to symptoms indicative of SARS-CoV-2 infection. PCR-testing was conducted according to current national guidelines and was not readily available in Sweden for non-hospitalized patients until spring-summer 2021. The severity of COVID-19 disease and the level of required treatment were recorded (see supplementary Table 1 for COVID-19 severity and required level of health care).
Figure 2. Description of method; study inclusion, collection of blood samples, serological analysis and statistical analysis.
Personnel were tested for anti-SARS-CoV-2 antibodies at three-time points during the study after receiving a general message via e-mail detailing voluntary inclusion in the study. Information from the medical records of personnel was not obtained in order to preserve the privacy and integrity of colleagues and therefore PCR-results of personnel could not be documented.
Both patients and personnel received information regarding their individual serological responses once the samples had been analysed.
Blood sample collection and laboratory analysis
Venous blood samples for serum preparation were collected using EDTA tubes (1 × 7 ml) at intervals described above. Following collection; samples were first handled at the Clinical chemistry lab at Akademiska sjukhuset (Uppsala, Sweden) and through an automated process they were centrifuged at 2400 g for seven minutes using a Teca 100 EVO/EVO3 machine. The plasma was then aliquoted, frozen, and transported to the Uppsala Biobank where it was stored at −80 °C until further analyses for serological signs of SARS-CoV-2 infection.
Serological analyses for SARS-CoV-2 specific IgM and IgG were conducted at the Zoonosis Science Center at Uppsala University, Sweden, using an immunoassay based on Luminex technology [Citation7]. Samples were classified as seronegative and seropositive based on Median Fluorescence Intensity (MFI) cut-offs: (i) seronegative: <300 MFI and (ii) seropositive: >800 MFI. Samples with an MFI between 300 and 800 were re-analysed and if MFI >300 in the second run these were considered seropositive.
Statistical analyses
To assess the association of seropositivity, PCR-positivity, anxiety or self-isolation and variables such as sex, age, self-isolation, cancer treatment, and birthplace, univariable and multivariable logistic regression was used. A multivariable model was constructed where the risk of becoming seropositive for patients that self-isolated was adjusted for gender, age (as a continuous variable), type of malignancy, treatment intention, place of birth, presence of children in the household and living situation (alone or with >1 person). Results are presented as odd ratios (OR) with a 95% confidence interval and a p-value. Tests with p < 0.05 were considered significant. The proportion of COVID-19 positive in subgroups of the cohort was compared using Chi-square test. Furthermore, time to seropositivity was illustrated using Kaplan-Meier curves and p-values for associated logrank tests are presented. Statistical analyses were performed using R version 4.2.2 [Citation8].
Results
Demographics
Out of 622 patients included in the study, the majority, 76% (n = 475), were treated for an SM while 24% (n = 147) had a HM (). The mean ages in these groups were comparable. SM patients were predominantly female (64%, n = 306), while a majority of HM patients were male (67%, n = 99). Two-thirds of all patients received treatment with palliative intention (67%, n = 415), similar for both SM and HM patients. Health care staff, n = 258, were younger and predominantly females (). The mean (SD) follow-up in days until the final serology sample was 206.5 (77.8) for SM patients, 237.1 (72.0) for HM patients and 200.1 (112.2) for healthcare personnel. The mean (SD) follow-up in days until seropositivity was 195.8 (82.2) for SM patients, 230.9 (78.1) for HM patients and 181.5 (113.2) for healthcare personnel.
Table 1. Clinical characteristics of 358 staff and 622 cancer patients included in the study, stratified by solid malignancy vs. hematological malignancy.
Seropositivity
Seropositivity was lower for SM patients, 10.5%, and HM patients, 6.8%, than for health care staff, 16.2% (p = 0.005, ). The time to reach SARS-CoV-2 seropositivity for patients and healthcare personnel is illustrated in a Kaplan–Meier plot ().
Figure 3. Patients with hematological malignancies (HM, red line) and patients with solid malignancies (SM, green line) had a lower degree of SARS-CoV-2 seropositivity than health care personnel (blue line) (logrank test p = 0.082).
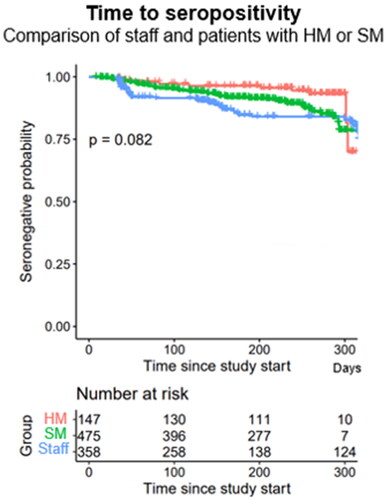
Table 2. Degree of anxiety vs. isolation and seropositivity, PCR-positivity and COVID-19-positivity in staff and patients; stratified by solid malignancy vs. hematological malignancy.
PCR- and seropositivity rates were similar for SM patients (11.4% vs. 10.5%, p = 0.75), while for HM patients the PCR-positivity rate was as expected numerically higher than seropositivity (10.2% vs. 6.8%, p = 0.3), although statistically non-significant (). PCR-positive HM patients more often required advanced in-patient treatment, including supplemental oxygen, non-invasive ventilation, or requiring treatment in an ICU (69%), compared to PCR-positive SM patients (69% vs 26%, p = 0.005), ().
Anxiety and self-isolation in patients
Moderate to high COVID-19-related anxiety was expressed by in total of 193 patients (32%, ). Patients with SM (n = 159, 34%) expressed higher anxiety than those with HM (n = 34, 25%) [OR = 1.6 (1.1–2.5), p = 0.04], but self-isolated to a similar degree, SM (n = 249, 54%) vs. HM (n = 80, 58%), [OR = 0.9 (0.6–1.3), p = 0.49]. The degree of anxiety or self-isolation at inclusion did not seem to vary among study participants over the inclusion time period as the pandemic continued (Supplemental Figure 1) and no significant difference in seropositivity was found when patients were stratified depending on the time of inclusion in the study; early inclusion (April 1st – May 11th) versus late inclusion (May 12th – August 1st), (Supplemental Figure 2).
A strong association was found between increasing age and increasing self-isolation [OR 1.03 (1.02, 1.05), p < 0.001], (Supplemental Table 2). A high degree of anxiety was more common among women [OR: 3.5, (2.4–5.2), p < 0.001] and patients born outside of Europe (as compared to patients born in Sweden) [OR: 2.9, (1.9–4.6), p = 0.007]. Patients who expressed high anxiety also self-isolated to a higher degree [OR: 2.8, (2.0–4.1), p < 0.001]. When comparing patients who expressed higher anxiety to those who self-isolated characteristics were overall similar, except for higher age being more common among those who self-isolated (Supplemental Table 3). In both groups, females tended to be more common among those who expressed a higher degree of anxiety and those who did self-isolate (p < 0.001).
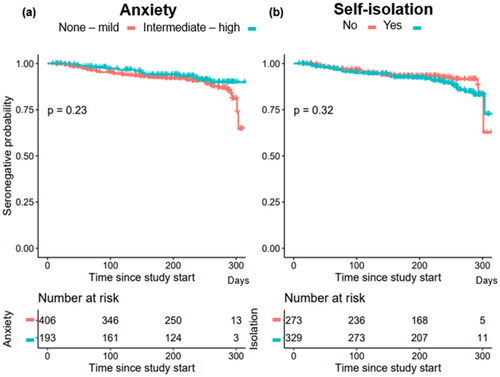
In univariable and multivariable analyses we also found no significant associations between age, gender, type of malignancy, treatment intention, place of birth, presence of children in the household or living situation and seropositivity (). Strict adherence to social distancing and self-isolation guidelines did not reduce the risk of becoming seropositive; univariable [OR 1.40 (0.81, 2.48), p = 0.23] and multivariable [OR: 1.67 (0.94, 3.04), p = 0.09]. The risk of becoming seropositive for patients with high anxiety was [OR: 0.66 (0.34, 1.21), p = 0.20]. Living alone or the presence of children in the household in the household did not significantly impact the risk of seropositivity in univariable ([OR 0.46 (0.19, 0.99), p = 0.07] and [OR 1.91 (1.01, 3.47), p = 0.04]) or multivariable analyses ([OR 0.55 (0.22, 1.22), p = 0.17] and [OR 1.54 (0.70, 3.33), p = 0.27]) respectively.
Table 3. Risk of seropositivity associated with degree of self-isolation gender, age, type of malignancy, treatment intention and place of birth.
Time to reach seropositivity for SARS-Cov-2 virus infection was next investigated; a high degree of anxiety and/or high degree of self-isolation at the time of answering the questionnaire did not influence the risk of seroconversion to becoming positive for SARS-Cov-2 virus infection ().
Discussion
The question of to what extent different levels of adherence to social distancing and self-isolation guidelines are protective of patients on active cancer treatment during a pandemic has to our knowledge not been previously examined. In this study of 622 Swedish patients undergoing active cancer treatment, high self-assessed COVID-19-related anxiety and/or strict adherence to social distancing and self-isolation guidelines at the beginning of the pandemic did not seem to decrease the risk of becoming SARS-CoV-2 seropositive later on. These results could be of importance when counselling patients required to regularly visit health care due to active treatment on protective measures in the event of a new pandemic.
Cancer itself is commonly associated with a heightened degree of anxiety [Citation9]. Information regarding measures to decrease the risk of infection is readily provided to patients on active cancer treatment. With the emergence of the COVID-19 pandemic, the main focus of anxiety shifted for many patients, with competing fears; either risk contracting an unknown and potentially fatal SARS-CoV-2-infection, or fear of delayed or missed cancer treatments due to pandemic guidelines or an infection [Citation6]. In the patient questionnaire in our study, participants provided personal comments reflecting both sentiments.
Infection-reducing practices have to be feasible to maintain over time in the context of a prolonged pandemic, as strict self-isolation guidelines could negatively impact a vulnerable group of patients’ ability to see their loved ones at a critical time of treatment. Therefore, it is important to address which approach is most suitable in order to reduce the risk of infection or progressive disease whilst not leading to unwarranted anxiety and isolation. In our study one third of patients displayed a high degree of anxiety regarding COVID-19, which corresponds to similar findings in a study by Moraliyage et al. [Citation10]. Neither a higher degree of anxiety nor adhering to Swedish self-isolation guidelines were statistically significantly associated with a lower risk of SARS-CoV-2 infection as depicted by seronegative probability throughout the time of the pandemic when vaccines were not available. However, in a multivariable model, a higher degree of anxiety indicated a trend for lower risk of SARS-CoV-2 seropositivity whilst employing strict self-isolation did not. Our results indicate that this population of cancer patients, on active treatment and with required regular health care appointments, was seemingly not wholly protected over time by the initial strategy and intent of a higher degree of isolation. It is therefore important to consider additional forms of protective measures when counselling patients on active cancer treatment, in the event of another global pandemic; that strict self-isolation on its own is not the sole strategy one needs to adopt, but rather as a part of a more varied approach.
It has been reported that having cancer, and especially if actively treated in conjunction with higher age [Citation2], increases the risk of a hospitalization due to COVID-19, but foremost that the incidence of SARS-CoV-2 infection with milder symptoms is higher among patients living with cancer [Citation11]. Furthermore, according to Lee et al. mortality from COVID-19 disease in cancer patients was not affected by cytotoxic treatment alone, but was instead driven by gender, age and other comorbidities [Citation2,Citation12–15], underlining the importance of continued anti-cancer treatment. Several studies have, however, also shown the opposite; that recent cytotoxic treatments increase the risk for adverse events and morbidity due to COVID-19 [Citation16,Citation17]. A complete lockdown may hypothetically protect against SARS-CoV-2 infection but seeing as this is not conducive to continued antitumoral treatment to prevent progressive disease (including necessary health care visits) it is not a feasible option, making community exposure probably unavoidable. The added stress associated with a complete lockdown can at least partially be avoided by employing a more nuanced risk-minimizing approach, allowing for more individual freedom and less strict isolation measures in the event of a new wave of this or another global pandemic. In this regard, our results support the Swedish approach [Citation18,Citation19] to the COVID-19 pandemic. The risk of severe COVID-19 has for most patient risk groups decreased with time, with the evolution of milder SARS-CoV-2-strains and the introduction of the effective vaccines. For cancer patients, however, the risk for severe disease remains; especially for patients treated with anti-CD 20 antibodies since they do not respond as well to the vaccines and have higher COVID-19-related mortality [Citation20,Citation21]. Other protective measures, such as access to antiviral therapy and supportive care, are therefore probably of more import than strict self-isolation for these individuals.
We found that for patients with SM, the PCR-positivity rate was comparable to seropositivity, while for patients with HM, a lower rate of seroconversion was found after PCR-positivity. Whether this was caused by the effects of anti-CD20-treatments, the nature of hematological malignancies, or other factors could not be determined in this study due to too few positive individuals. However, when examining the course of infections among the patients with PCR-positive tests it was more common that HM-patients required more advanced in-patient care compared to PCR-positive SM-patients, as has previously been seen in studies both from our group and others [Citation22–24].
The degree of seropositivity among personnel was higher than among patients throughout the study period, covering the first and second waves of the pandemic, similar to results found in studies conducted in France [Citation25].
Women and patients born outside of Europe expressed more anxiety. Patients born outside of Europe were more adversely affected by the pandemic during the study period compared to individuals born in Europe, in terms of a higher number of confirmed cases, patients requiring intensive care and a higher number of fatalities [Citation26]. Others have also shown that female patients with curative cancer treatment are more likely to suffer from COVID-19-related anxiety [Citation27]. In the general population women commonly express a higher degree of anxiety, probably explaining the gender difference seen in our study, as knowledge that men were more at risk of severe COVID-19 was well known early in the pandemic [Citation2].
Strengths and limitations
A strength of the study is the prospective design of the serological testing with inclusion of a fairly large number of patients on active cancer treatment, representative of a geographical healthcare region. That patients have been followed longitudinally, combined with detailed data on patient characteristics, PCR-testing, and recording of COVID-19 severity from electronic health records adds to the strengths of the study.
A significant limitation however is that the availability of SARS-CoV-2 PCR-testing was severely limited during the early phase of the study period, leading to a sampling bias of only severe cases of COVID-19 being confirmed with PCR during that time. PCR-testing was mainly restricted to those patients in need of in-hospital treatment at the beginning of the study period but became more generally available and recommended for all individuals with symptoms indicative of COVID-19 toward the end of the study. Furthermore, as described previously, PCR-testing was not conducted on health care personnel due to the current guidelines in effect, and information regarding such results could not be obtained from medical records of personnel in order to preserve their integrity. Another limitation is that, to protect the patients from the risk of increased exposure, we decided to only perform serological testing at intervals determined by regular planned health care contacts, and not at the same intervals for all patients.
Finally, a limitation of this study is that the self-reported degree of anxiety and self-isolation was tested at the time of inclusion and not later on during the pandemic when these attitudes could have changed; these exposures were thus considered to remain over time for these patients when analyzing serological response.
Conclusion
In a study of 622 Swedish patients with active cancer treatment, an initially high degree of COVID-19 related anxiety and strict adherence to social distancing and self-isolation guidelines at the beginning of the pandemic were not associated with a lower risk of later developing COVID-19 seropositivity.
Although patients with hematological malignancies and COVID-19 were less likely to develop an antibody response and more likely to require advanced hospital care, they expressed less COVID-19-related anxiety than patients with solid malignancies.
Ethics approval
This study was approved by the Regional Ethics Committee in Uppsala (2010/98, 2020-01781).
Supplemental Material
Download MS Word (789.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data generated during the current study are not publicly available due to regulations on personal data protection but are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Ethics__COVID-19__Restrictive_Measures_-_Apr_14.pdf. [cited 2022 Sep 1]; Available from: https://media.tghn.org/articles/Ethics__COVID-19__Restrictive_Measures_-_Apr_14.pdf.
- Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19–infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021; 113(4):371–380. doi: 10.1093/jnci/djaa168.
- Zarifkar P, Kamath A, Robinson C, et al. Clinical characteristics and outcomes in patients with COVID-19 and cancer: a systematic review and meta-analysis. Clin Oncol. 2021;33(3):e180–91–e191. doi: 10.1016/j.clon.2020.11.006.
- Uppdrag att löpande se över och vid behov uppdatera sammanställningen över de identifierade grupperna som löper störst risk att drabbas av särskilt allvarlig sjukdomsutveckling vid insjuknande i covid-19 (S2021/00825 delvis). [cited 2023 Feb 13]; Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/dokument-webb/ovrigt/socialstyrelsen-delredovisning-riskgrupper-covid-19.pdf.
- Förekomsten av antikroppar mot SARS-CoV-2 i Sverige, 26 April – 9 May 2021. [cited 2022 Sep 1]; Available from: https://www.folkhalsomyndigheten.se/contentassets/45eafde72689438a8a21efa93a5591a4/forekomsten-antikroppar-mot-sars-cov-2.pdf.
- Erdoğan AP, Ekinci F, Acar Ö, et al. Level of COVID-19 fear in cancer patients. Middle East Curr Psychiatry. 2022;29(1):9. doi: 10.1186/s43045-022-00181-5.
- Hoffman T, Kolstad L, Lindahl JF, et al. Diagnostic potential of a luminex-based coronavirus disease 2019 suspension immunoassay (COVID-19 SIA) for the detection of antibodies against SARS-CoV-2. Viruses. 2021;13(6):993. doi: 10.3390/v13060993.
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Internet]. 2021. Available from: https://www.R-project.org/
- Carey LB, Cohen J, Koenig HG, et al. COVID-19, mental health and cancer. J Relig Health. [Internet]. 2021; Aug [cited 2022 Jun 2]60(4):2191–2195. Available from: https://link.springer.com/10.1007/s10943-021-01318-2 doi: 10.1007/s10943-021-01318-2.
- Moraliyage H, De Silva D, Ranasinghe W, et al. Cancer in lockdown: impact of the COVID-19 pandemic on patients with cancer. Oncologist. 2021;26(2):e342–4–e344. doi: 10.1002/onco.13604.
- Lee KA, Ma W, Sikavi DR, et al. Cancer and risk of COVID-19 through a general community survey. Oncologist. 2021;26(1):e182–5–e185.
- Rogiers A, Pires da Silva I, Tentori C, et al. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9(1):e001931. doi: 10.1136/jitc-2020-001931.
- Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9.
- Madan A, Siglin J, Khan A. Comprehensive review of implications of COVID‐19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic. Cancer Med. 2020;9(24):9205–9218. doi: 10.1002/cam4.3534.
- Brar G, Pinheiro LC, Shusterman M, et al. COVID-19 severity and outcomes in patients With cancer: a matched cohort study. J Clin Oncol. 2020;38(33):3914–3924. doi: 10.1200/JCO.20.01580.
- Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. 2020;141:92–104. doi: 10.1016/j.ejca.2020.09.028.
- Chavez-MacGregor M, Lei X, Zhao H, et al. Evaluation of COVID-19 mortality and adverse outcomes in US patients With or Without cancer. JAMA Oncol. 2022;8(1):69–78. doi: 10.1001/jamaoncol.2021.5148.
- Ludvigsson JF. The first eight months of Sweden’s COVID‐19 strategy and the key actions and actors that were involved. Acta Paediatr. 2020; 109(12):2459–2471. doi: 10.1111/apa.15582.
- Baral S, Chandler R, Prieto RG, et al. Leveraging epidemiological principles to evaluate Sweden’s COVID-19 response. Ann Epidemiol. 2021;54:21–26.
- Patel NJ, D'Silva KM, Hsu TY-T, et al. Coronavirus disease 2019 outcomes among recipients of anti‐cd20 monoclonal antibodies for immune‐mediated diseases: a comparative cohort study. ACR Open Rheumatol. 2022;4(3):238–246. doi: 10.1002/acr2.11386.
- Liebers N, Speer C, Benning L, et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients. Blood. 2022;139(1):142–147. doi: 10.1182/blood.2021013445.
- Larfors G, Pahnke S, State M, et al. Covid-19 intensive care admissions and mortality among swedish patients with cancer. Acta Oncol. 2021; 60(1):32–34. doi: 10.1080/0284186X.2020.1854481.
- Ullgren H, Camuto A, Rosas S, et al. Clinical characteristics and factors associated with COVID-19-related death and morbidity among hospitalized patients with cancer: a Swedish cohort study. Acta Oncol. 2021; 60(11):1459–1465. doi: 10.1080/0284186X.2021.1958005.
- Pagano L. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. doi: 10.1186/s13045-021-01177-0.
- Ladoire S, Rederstorff E, Goussot V, et al. Parallel evolution and differences in seroprevalence of SARS-CoV-2 antibody between patients with cancer and health care workers in a tertiary cancer Centre during the first and second wave of COVID-19 pandemic: canSEROcov-II cross-sectional study. Eur J Cancer. 2022;165:13–24. doi: 10.1016/j.ejca.2022.01.005.
- Utrikesfödda och covid-19. Konstaterade fall, IVA-vård och avlidna bland utrikesfödda i Sverige. [cited 2022 Sep 1]. Available from: https://www.folkhalsomyndigheten.se/publikationer-och-material/publikationsarkiv/u/utrikesfodda-och-covid-19/.
- Sigorski D, Sobczuk P, Osmola M, et al. Impact of COVID-19 on anxiety levels among patients with cancer actively treated with systemic therapy. ESMO Open. 2020;5(5):e000970. doi: 10.1136/esmoopen-2020-000970.