Abstract
Background
Several new systemic treatments for primary metastatic prostate cancer patients (mPCa) were introduced in the last decade for both hormone-sensitive (mHSPC) and castration-resistant prostate cancer (mCRPC). However, little is known about the introduction of these treatments in clinical practice. In this national cohort study, we described users and non-users of systemic treatment beyond androgen deprivation therapy (ADT). We also explored whether there was a shift in treatment patterns after the introduction of Docetaxel for mHSPC patients.
Materials and Methods
All patients registered in the Cancer Registry of Norway with mPCa diagnosed in 2010-18 were included. Data on systemic therapy (Docetaxel, Abiraterone, Enzalutamide, Cabazitaxel, and Radium-223) were provided from the Norwegian Prescription Database, the Norwegian Patient Registry, and the Norwegian Control and Payment of Health Reimbursement Database. Descriptive results about patient and disease characteristics were presented using frequencies and proportions, means and standard deviations, or medians and interquartile ranges.
Results
Of the 2770 patients included in this study, 48% received systemic treatment beyond ADT. The proportion of patients receiving systemic treatment increased during the study period. Systemic treatment users were younger, in better general condition, and had more aggressive tumors than non-users. A treatment shift was observed after 2015, with 48% of patients receiving systemic treatment (mainly Docetaxel) in the mHSPC phase compared to 4% of those diagnosed 2010-14. No significant treatment differences were observed across health regions.
Conclusions
An increasing proportion of patients received systemic treatment during the period 2010–18. However, less than 50% of patients in our study received systemic treatment. In accordance with updated guidelines, Docetaxel was introduced after 2015 with an increasing proportion of patients receiving systemic treatment as mHSPC. Further studies should address the disease course and treatment given to patients who do not receive systemic treatment.
Background
Prostate cancer (PCa) is the second most common cancer among men [Citation1]. About 10% of patients diagnosed with PCa have distant metastases at diagnosis (mPCa) and androgen deprivation therapy (ADT) has long been standard treatment for these patients [Citation2,Citation3]. Docetaxel was introduced almost 20 years ago for patients with metastatic castration resistant PCa (mCRPC) [Citation4,Citation5]. Several new treatments have been introduced in the past 10 years for mCRPC, and Abiraterone, Enzalutamide, Radium-223 and Cabazitaxel were subsequently made available in the Norwegian public health care system. All systemic treatment except ADT was initially restricted to mCRPC patients. However, in 2015, a survival gain was demonstrated in clinical studies for patients with hormone sensitive metastatic prostate cancer (mHSPC) who received Docetaxel upfront, which changed treatment guidelines for patients with primary mPCa [Citation6,Citation7].
Several population-based studies have documented the use of systemic treatment for mCRPC patients and Docetaxel in mHSPC patients in clinical practice. However, real-world studies of mCRPC patients often include both primary and secondary metastatic patients. Moreover, few mCPRC and mHSPC studies have focused on patients who did not receive systemic treatment beyond ADT [Citation8–14].
This population-based cohort study describes the patient characteristics and treatment patterns for patients diagnosed with mPCa in Norway during 2010–2018. Specifically, we described all patients – both those who received only ADT and those who received further systemic treatment – with respect to clinical, geographic, and socioeconomic features. We also presented the use of systemic treatment, time from diagnosis to start of first systemic treatment, and assessed whether there was a treatment shift for mHSPC after publications recommended Docetaxel for patients with this disease.
Materials and methods
Study population
Our study population comprised all men registered in the Cancer Registry of Norway (CRN) with histologically verified adenocarcinoma and distant metastases diagnosed in Norway between 2010 and 2018 (ICD-10 C61). We excluded records with missing information about metastasis and patients diagnosed with mPCa at autopsy.
Data sources
This study used data from the population-based CRN including the Prostate Cancer Registry (NoPCR), a clinical quality registry. From these registries, we used information about age, diagnosis date, prostate-specific antigen (PSA) at diagnosis (ng/ml), Gleason score at biopsy, clinical TNM, as well as treatment data about surgery and radiation therapy [Citation15]. WHO performance status as a measure of functional status at diagnosis was also obtained [Citation16]. From the Norwegian Prescription Database (NorPD), we used information about prescription systemic treatments dispensed in Norway including dispensing date. From the Norwegian Patient Registry (NPR), we used information about chemotherapy use (Docetaxel, Cabazitaxel, and unspecified chemotherapy). The Norwegian Control and Payment of Health Reimbursement Database (KUHR) provided information about all Radium-223 infusions given. Statistics Norway (SSB) provided data about household income and highest obtained education.
The following variables were categorized: PSA at diagnosis (≤100 mg/ml or >100mg/ml), tumour localization at diagnosis (localized: cT0-cT2 or locally advanced: cT3-cT4), Gleason score (6–7 or 8–10), clinical lymph node status (negative or positive), WHO status at diagnosis (0, 1–2, or 3–4), highest obtained education (none, elementary, high, or vocational school; or college or university), and household income in tertiles (1, 2, or 3). The Appendix contains additional details on how income tertiles were calculated.
Drug use
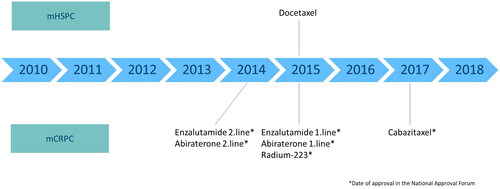
After approval by the Norwegian Medicines Agency, almost all cancer therapy in Norway is provided by the public health care system [Citation16]. Abiraterone [Citation17] and Enzalutamide [Citation18] were initially approved for second line treatment after Docetaxel for mCRPC (). In 2015, both drugs were approved for first line treatment and Docetaxel was introduced for mHSPC [Citation19,Citation20]. Radium-223 has also been available for clinical practice in Norway outside clinical trials since 2015 [Citation21]. Cabazitaxel was approved for mCRPC in 2017 [Citation22].
In this study, 98% of “users” received ADT and we therefore defined additional mPCa treatment with Abiraterone, Enzalutamide, Cabazitaxel, Radium-223, or Docetaxel as systemic treatment. Furthermore, because clinical recommendations changed in 2015 when Docetaxel was introduced for mHSPC, we described treatment before and after 2015 separately.
Users were in this study defined as patients who received systemic treatment, which was defined as meeting at least one of the following criteria:
collected a prescription of Abiraterone or Enzalutamide
received at least one infusion of Docetaxel, Cabazitaxel, or Radium-223
All other patients were defined as non-users.
Because we did not have information on PSA after diagnosis, we used time since diagnosis to distinguish between treatment given in the hormone sensitive and castration resistant phases. We assumed that patients who received systemic treatment ≤6 months after diagnosis were mHSPC patients and that patients who received systemic treatment >12 months after diagnosis were mCRPC patients. Patients who received systemic treatment >6 to 12 months after diagnosis were categorized as “in between”.
Statistics
Descriptive results about patient and disease characteristics for the PCa patients were presented using frequencies and proportions, means and standard deviations, or medians and interquartile ranges.
To avoid bias from non-random missing information in multivariable analyses, we performed multiple imputation with chained equations [Citation23]. Results using imputed data were based on 10 imputed datasets and patients who had still missing data after imputation were excluded (n = 5). Details about the imputation models are provided in the Appendix. Patient and disease characteristics based on the imputed data (Supplementary Table S1) were generally similar to those obtained from the observed data.
Unadjusted differences in characteristics between users and non-users were evaluated from the observed data using the two-sided Chi-square test (binary and nominal variables) or the two-sided, two sample Wilcoxon rank-sum test (ordinal variables and age). Adjusted differences were evaluated from the imputed data using a logistic regression model that included all patient and disease characteristics, diagnostic period (2010–2014 or 2015–2018), and health region (South-Eastern, Western, Central, or Norther) as covariates. Age was modelled using restricted cubic splines.
Systemic treatment use among mHSPC, “in-between”, and mCRPC patients was described using frequencies and proportions and stratified by diagnostic period. We estimated the cumulative incidence of becoming a user up to 5 years after diagnosis, where death was considered a competing risk. Observations were censored on 31 December 2018. The observed cumulative incidence was presented graphically and stratified by year of diagnosis: 2010–2014, 2015, 2016, and 2017; results were not shown for patients diagnosed in 2018 due to the short follow-up time. Time from diagnosis to treatment start was also presented using the median and interquartile range.
All analyses were performed using Stata (version 17.0) except a Sankey diagram created using R (version 4.1.3) using the dplyr (version 1.1.1) and networkD3 (version 0.4) packages [Citation24–26]. Cumulative incidences were calculated using the stcompet package for Stata [Citation27].
Results
Patient characteristics
During 2010–2018, 2770 patients were diagnosed with primary mPCa in Norway. The median follow-up time in our study was 24.7 months (IQR 11.6 to 43.7 months, range 0 to 107 months). Of these patients, 48% (n = 1327) were defined as users of systemic treatment and 52% (n = 1443) were defined as non-users (). Users were younger at diagnosis than non-users with a median age of 69 years compared to 77 years. Users presented with more aggressive tumor characteristics at diagnosis than non-users: median PSA was 100 vs 69 (ng/ml), 75% vs 66% of patients presented with a T3-4 tumor, and 70% vs 50% had a Gleason score ≥8. Additionally, 27% of users had education above vocational school level compared to 18% among non-users. A greater proportion of users had a high income (27% of users compared to 16% of non-users), and a higher proportion of users had a WHO status of 0–2 compared to non-users (86% vs 73%). The aforementioned differences between users and non-users were all significant with p < 0.001. Investigating patient characteristics between users and non-users stratified by diagnostic period revealed similar results (Supplementary Table S2).
Table 1. Patient and disease characteristics at diagnosis.
The proportion of users and non-users were approximately the same across health regions; 53% of users and 55% of the non-users were from the Southeast region ().
Treatment regimens
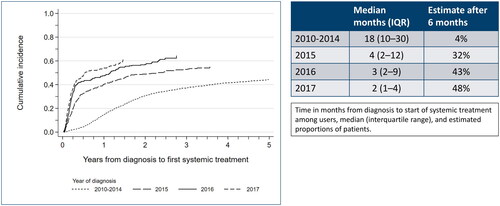
illustrates the proportion of users over time (cumulative incidence), stratified by year of diagnosis. Overall, a higher proportion of mPC patients received systemic treatment in each subsequent diagnosis period. Moreover, patients diagnosed in 2015–17 received systematic treatment sooner after diagnosis than patients diagnosed in 2010–14. While few (4%) mHSCP patients diagnosed between 2010-14 received systemic treatment within 6 months after their diagnoses, 32%/43%/48% of mHSCP patients received systemic treatment within 6 months when diagnosed in 2015/16/17, respectively. The earlier introduction of systemic treatment in later diagnostic years was also reflected by a decreasing time until first systemic treatment use (median time to first use: 18 months in 2010-14 versus 2–4 months in 2015–2017). Patients diagnosed late in 2018 would not have had sufficient time to start treatment with Docetaxel and were therefore excluded from this analysis and .
Figure 2. Cumulative proportion of systemic treatment use (ever-use) since diagnosis among men diagnosed with metastatic prostate cancer during 2010–2017.
describes the number and percentage of mPCa patients receiving specific systemic treatments before and after the treatment shift in 2015, taking into consideration time since diagnosis. Docetaxel was the most frequently used first line drug for mHSPC (89%) and mCRPC patients (42%) diagnosed between 2010–14. In line with treatment guidelines, Docetaxel was the primary treatment for 96% of mHSPC patients diagnosed after 2015, and newer systemic treatments were increasingly used for mCRPC patients diagnosed in this period (Enzalutamide 69% and Abiraterone 12%).
Table 2. Start of first systemic treatment by time after diagnosis, stratified by diagnostic period. Numbers of patients and percentages are reported n (%).
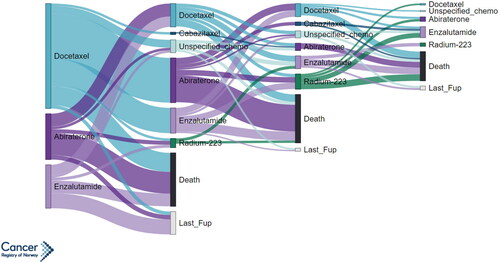
illustrates the first four systemic treatment lines for patients diagnosed with primary mPCa during 2010–14. Of the 744 patients who received 1st line systemic treatment, 61% received 2nd line therapy, 31% received 3rd line therapy, and 13% received 4th line therapy. Among patients who received two lines of systemic treatment, those who received Docetaxel as first line treatment were likely to receive Abiraterone/Enzalutamide as second line treatment (85% of first line Docetaxel users). The opposite was also observed where those who received Abiraterone/Enzalutamide as first line treatment were likely to receive Docetaxel as second line treatment (89% of first line Abiraterone/Enzalutamide users). Systemic treatment lines were not illustrated for patients diagnosed after 2015 since many of these patients did not receive more than first line systemic treatment during the study period.
Figure 3. Sankey diagram showing treatment pathways starting from first use of systemic treatment for men diagnosed with metastatic prostate cancer in Norway during 2010–2014. The width of treatment pathways is proportional to the number of patients who received a given treatment; pathways are only shown for groups of 5 or more.
Discussion
We observed that less than 50% of mPCa patients in this study received systemic treatment, but that there was a trend towards increased use of systemic treatment during the study period (2010-18). A shift in treatment pattern started in 2015, after which more patients received systemic treatment (Docetaxel in particular) in mHSPC phase. Compared to non-users, users of systemic treatment were younger, had a better general condition, and had more aggressive tumour characteristics at diagnosis.
The use of systemic treatment beyond ADT increased during 2010–18. Overall, mPCa patients have a fairly good prognosis and many of those diagnosed in 2010–14 were still alive to receive new systemic treatments after these drugs were approved. For patients diagnosed in 2015 and later, Docetaxel was indicated upfront, and a larger proportion of patients started systemic treatment in the mHSPC phase. However, we could have expected the proportion of users in our study to be even higher than observed from 2015 onwards as these patients most likely are in better condition in the mHSPC phase than in the mCRPC phase and should therefore be eligible for systemic treatment. Nevertheless, clinicians might have been more restrictive in prescribing Docetaxel because the indication was new. Patients diagnosed in more recent years who did not receive treatment in the mHSPC phase may not have reached the mCRPC phase and the proportion of users in this group is likely to increase with longer follow-up. The varying ratios between Abiraterone and Enzalutamide is a result of changes in reimbursement in the Norwegian public health system.
The proportion of users of systemic treatment among mCRPC patients in our study (48%) is in line with previously published studies. A Swedish study of mCRPC patients published in 2013 (when only Docetaxel was available) found that 61% of patients under 70 years old and 30% of patients between 70–79 received chemotherapy [Citation28]. In a more recent Swedish study including primary and secondary mCRPC patients, 36% of mCRPC patients received systemic treatment, increasing to 50% in 2013–2015 [Citation29]. In a Dutch study including patients diagnosed during 2010–13, a higher proportion of trial patients (ever) received Docetaxel compared to non-trial patients (85% vs 40%) [Citation30]. Prospective studies such as the Treatment Registry for Outcomes in CRPC Patients (TRUMPET) will give us a better understanding of the treatment for mCRPC patients in clinical practice [Citation31].
During 2010-18, the introduction of several new systemic therapies for mCRPC and Docetaxel for mHSPC changed the treatment of patients with mPCa. We observed a change in Norwegian treatment practice after 2015, which was soon after Docetaxel was shown to give a survival benefit for mHSPC [Citation7,Citation32]. For patients diagnosed during 2010–2014, only 10% of those who received systemic treatment did so during the mHSPC phase compared to 73% of patients diagnosed in 2015-18. This indicates that international guidelines were successfully implemented in Norway. Median time from diagnosis to systemic treatment decreased from 18 months for patients diagnosed in 2010-14 to 2 months for patients diagnosed in 2017.
New drugs for mPCa are typically expensive and there has been a focus on whether all patients have equal access to therapy. The Norwegian Approval Forum was established in 2013 to ensure equal access to expensive treatments in Norway. In our study, the proportion of users was fairly similar across the four health regions (about 50% users in each region) which suggests there is equal access to these drugs across Norway, and also suggests that similar treatment strategies are being used throughout the country in a broad sense (our sample was too small to stratify results by hospital). However, the proportion of patients with high level of education and/or high income is greater among users than non-users, which suggests that there is some inequality in the use of systemic treatment. Inequal use of systemic treatment might not be directly related to education or income but instead related to other, non-recorded variables associated with education level and income.
As we did not have information about PSA/testosterone levels after diagnosis, we were not able to identify when patients reached castration resistant status. We assumed that all patients had hormone sensitive disease the first six months after diagnosis even though a small portion of these patients would have been primary mCRPC because the impact of this misclassification bias was assumed to be minimal. Additionally, because patients who received systemic therapy 6–12 months after diagnosis could be “late” HSCP or “early” CRPC, we classified them in a separate “in between” group to avoid additional misclassification in the mHSPC and mCRPC groups (). Patients receiving systemic treatment >12months after diagnosis are most likely mCRPC so the risk of misclassification was least in this group. We could have used the time of systemic treatment start as a surrogate for mCRPC for patients diagnosed when systemic treatment was only indicated for mCRPC (2010-14). However, the observed median of 1.5 years from diagnosis to start of systemic treatment/mCRPC in our study was longer than previously reported. Patients in our study may have therefore reached a castration resistant phase prior to approval of these drugs and were only able to receive them after their approval. Using this approach would have biased (prolonged) the observed time from diagnosis to start of systemic treatment treatment/castration resistant phase and would therefore also cause misclassification.
Strengths and limitations
This population-based registry study includes all patients diagnosed with mPCa in Norway during 2010–18 and used individual data on systemic treatment. The patient group represents a real-world setting and all patients had primary mPCa. Moreover, our study also describes mPCa patients who did not receive systemic treatment, something few previous studies have done.
As with other registry-based studies, our study was limited by the variables collected at the CRN. In particular, we did not have detailed information about when patients became castration resistant and did not have any additional information about why certain patients remained non-users. In our study 98% of users received ADT. ADT is indicated for all mPCa patients and few exceptions could be expected among users of systemic treatment. However, information on ADT given at hospitals was not available for this study, there might be some cases where guidelines are not followed, and we cannot exclude some misclassification in the CRN.
We had limited follow-up data to describe second line treatment and survival for patients diagnosed in 2015–18, including patients treated with Docetaxel as mHSPC. Since 2018, several new drugs have been introduced for mHSPC and mCRPC. Our data will be updated in the near future, giving us the opportunity to present further changes in treatment and survival for patients with mPCa.
Conclusion
Nearly 50% of primary mPCa patients received systemic treatment, with an increase in the proportion of users after introduction of new androgen receptor target and Docetaxel for mHSPC. The proportion of users will probably increase with longer follow-up. At diagnosis, users were younger, in better general condition and had more aggressive tumors compared to non-users. Docetaxel was introduced after 2015 for mHSPC according to international guidelines. No significant differences in the proportion of users were observed between health regions.
The data that support the findings of this study are available from the corresponding author (AHS), upon reasonable request.
Supplemental Material
Download MS Word (30.7 KB)Supplemental Material
Download MS Word (25.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Norway CRo. Cancer in Norway 2019 - Cancer incidence, mortality, survival and prevalence in Norway. 2020.
- Pagliarulo V, Bracarda S, Eisenberger MA, et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol. 2012;61(1):11–25. doi: 10.1016/j.eururo.2011.08.026.
- Fossa SD, Jacobsen AB, Ginman C, et al. Weekly docetaxel and prednisolone versus prednisolone alone in androgen-independent prostate cancer: a randomized phase II study. Eur Urol. 2007;52(6):1691–1698. doi: 10.1016/j.eururo.2007.01.104.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720.
- James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol. 2015;67(6):1028–1038. doi: 10.1016/j.eururo.2014.09.032.
- Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747.
- Chowdhury S, Bjartell A, Lumen N, et al. Real-world outcomes in first-line treatment of metastatic castration-resistant prostate cancer: the prostate cancer registry. Target Oncol. 2020;15(3):301–315. doi: 10.1007/s11523-020-00720-2.
- Shayegan B, Wallis CJD, Malone S, et al. Real-world use of systemic therapies in men with metastatic castration resistant prostate cancer (mCRPC) in Canada. Urol Oncol. 2022;40(5):192.e1–e9. doi: 10.1016/j.urolonc.2022.01.009.
- Wen L, Valderrama A, Costantino ME, et al. Real-world treatment patterns in patients with castrate-resistant prostate cancer and bone metastases. Am Health Drug Benefits. 2019;12(3):142–149.
- Anton A, Pillai S, Semira MC, et al. Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer. BJUI Compass. 2022;3(3):205–213. doi: 10.1002/bco2.129.
- Lavoie JM, Zou K, Khalaf D, et al. Clinical effectiveness of docetaxel for castration-sensitive prostate cancer in a real-world population-based analysis. Prostate. 2019;79(3):281–287. doi: 10.1002/pros.23733.
- Isaksson J, Green H, Papantoniou D, et al. Real-world evaluation of upfront docetaxel in metastatic castration-sensitive prostate cancer. World J Clin Oncol. 2021;12(11):1009–1022. doi: 10.5306/wjco.v12.i11.1009.
- Lendorf ME, Petersen PM, Svendsen AS, et al. Effectiveness of docetaxel for metastatic hormone-sensitive prostate cancer in clinical practice. Eur Urol Open Sci. 2021;24:25–33. doi: 10.1016/j.euros.2020.12.006.
- Hernes E, Kyrdalen A, Kvåle R, et al. Initial management of prostate cancer: first year experience with the norwegian national prostate cancer registry. BJU Int. 2010;105(6):805–811; discussion 811. doi: 10.1111/j.1464-410X.2009.08834.x.
- NYE METODER https://nyemetoder.no/en.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X.
- Multiple-Imputation Reference Manual, version 18: Stata Press; 2021. 388 p.
- A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria: R Core Team; 2022.
- Wickham H, Fr Henry L. Müller K dplyr: A grammar of data manipulation. 2022 [cited 2022 Dec 5]. Avaialble: https://dplyr.tidyverse.org., https://github.com/tidyverse/dplyr.
- Allaire JE, Gandrud C, Kuo K, et al. Yetman CJ networkD3: D3 JavaScript Network Graphs [cited 2022 Dec 5]. from R. R package version 0.4. https://CRAN.R-project.org/package=networkD3. 2017
- Coviello V, Boggess MA. Cumulative incidence estimation in the presence of competing risks. The Stata Journal. 2004;4(2):103–112. doi: 10.1177/1536867X0400400201.
- Lissbrant IF, Garmo H, Widmark A, et al. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013;52(8):1593–1601. doi: 10.3109/0284186X.2013.770164.
- Vigneswaran HT, Warnqvist A, Andersson TML, et al. Real world treatment utilization patterns in patients with castration-resistant prostate cancer. Scand J Urol. 2021;55(4):299–306. doi: 10.1080/21681805.2021.1936626.
- Westgeest HM, Uyl-de Groot CA, van Moorselaar RJA, et al. Differences in trial and real-world populations in the dutch castration-resistant prostate cancer registry. Eur Urol Focus. 2018;4(5):694–701. doi: 10.1016/j.euf.2016.09.008.
- Penson DF, Lin DW, Karsh L, et al. Treatment registry for outcomes in patients with castration-resistant prostate cancer (TRUMPET): a methodology for real-world evidence and research. Future Oncol. 2016;12(23):2689–2699. doi: 10.2217/fon-2016-0298.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi: 10.1016/S0140-6736(15)01037-5.