To the Editor
The use of immune checkpoint inhibitor (ICI) therapy in cancer treatment has improved patient outcomes by increasing the immune response to fight tumor cells [Citation1]. However, over-activation of the immune response is also responsible for immune-related adverse events (irAEs) [Citation2]. Cutaneous irAEs occur in up to 30% of patients treated with ICIs and encompass a wide range of events including lichenoid reactions, eczema, vitiligo, and pruritus () [Citation4]. Although cutaneous irAEs secondary to ICIs are common, they can vary in presentation and may go underdiagnosed or underreported. There has been only one report of lymphomatoid papulosis (LyP) secondary to an ICI in a patient with renal cell carcinoma [Citation4,Citation5]. Additionally, there has been one report of CD30+ cell infiltrate secondary to an ICI in a patient with stage IV metastatic melanoma, for which malignancy was excluded but no formal diagnosis was made [Citation6]. There have been no reports of LyP secondary to an ICI in a patient with Hodgkin lymphoma or an autologous stem cell transplant (autoSCT). Here we present a case of a patient with recent autoSCT for Hodgkin lymphoma taking ICIs who developed ulcerative LyP.
Table 1. Cutaneous immune-related adverse events (irAEs) secondary to immune checkpoint inhibitors (ICIs) and differential diagnoses [Citation13].
Case report
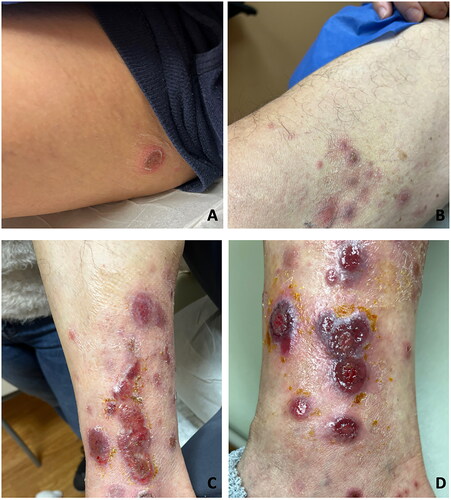
A 63-year-old male with recurrent Hodgkin lymphoma, initiated on treatment with nivolumab and brentuximab and followed by autoSCT 2 months later, presented to dermatology 2 months after autoSCT with 2 weeks of mildly painful leg ulcers. Nivolumab was held due to rash. The lesions began as papules that evolved into blisters, some pus-filled and ruptured into ulcers. The patient denied systemic symptoms and recent travel. Examination revealed grouped pink firm nodules on the right medial thigh, scattered round crusted plaques on the bilateral thighs, and grouped red ulcerated papules and plaques with a violaceous rim on the right medial leg (). Differential diagnoses included lymphoma cutis, LyP, neutrophilic dermatosis, bullous/erosive lichen planus, and infection.
Figure 1. Pink firm nodules, round crusted plaques, erosions with violaceous rim at initial presentation (A–C) and 2 weeks after initial presentation (D). A) Right groin. B) Right thigh. C) Right medial ankle. D) Right medial ankle.
A punch biopsy demonstrated a superficial and deep perivascular and interstitial mixed inflammatory cell infiltrate with many enlarged lymphocytes. Immunohistochemical staining of the infiltrate consisted of mostly CD3+ T-cells with a predominance of CD4 over CD8. The large lymphocytes expressed CD30 but not CD15 or ALK1. A T-cell receptor gene rearrangement study demonstrated evidence of monoclonal T-cell proliferation. A separate punch biopsy was performed for tissue cultures, which were negative for bacteria, mycobacteria, and fungi. Histopathology and clinical presentation were consistent with a diagnosis of LyP, however, the presence of activated T-cell lymphocytes was unusual. This histopathology differed from the patient’s prior histopathology on diagnosis of Hodgkin lymphoma, in which large Reed Sternberg cells positive for both CD30 and CD15 were identified. The patient was managed with supportive wound care with improvement, nivolumab was resumed 6 weeks after the initial presentation, and resolution was achieved 8 weeks after the initial presentation without LyP recurrence.
Discussion and conclusion
There is a spectrum of primary cutaneous and systemic CD30+ lymphoproliferative diseases linked by the expression of CD30+ tumor cells [Citation7,Citation8]. This spectrum includes benign and premalignant diseases, such as LyP, and malignancies such as anaplastic large-cell, B-cell, T-cell, and classical Hodgkin lymphoma [Citation7,Citation8]. LyP is a benign, chronic, CD30+ cutaneous lymphoproliferative disorder that is characterized by crops of papules, plaques, or nodules that often self-resolve and can recur [Citation9]. Given our patient’s clinical and histologic features, our patient was diagnosed with ulcerative LyP potentially occurring as a reaction to immune checkpoint inhibitor exposed T-lymphocytes from nivolumab therapy later activated by autoSCT.
Little is known about the dynamics of PD-1 expression on immune effector cells after SCT, however, there is evidence that PD-1 inhibition improves the antitumor effect of transplantation procedures [Citation10]. Malignant cells can compromise the antitumor effects of SCT by misusing the self-limiting system of the immune response by overexpressing inhibitory molecules that interact with immune cells, resulting in immune exhaustion [Citation11]. ICIs prevent the activation of the immune system in order to prevent autoimmunity and maintain peripheral tolerance. Nivolumab inhibits a transmembrane protein known as PD-1 that is expressed on T-cells, B-cells, and NK-cells [Citation11]. The ligand of PD-1 is PD-1L and is not only expressed on hematopoietic and non-hematopoietic cells but also on various solid tumor and hematologic cancer cells [Citation11]. The use of ICIs should decrease the activation of T-cell lymphocytes, thereby decreasing the likelihood of immune exhaustion [Citation11]. Our patient was first treated with ICIs, inhibiting T-cell activation, followed by autoSCT, which we believe activated the T-cells that were previously inhibited by nivolumab and resulted in a cutaneous irAE. These activated T-cells were seen on histology in our patient who was diagnosed with LyP secondary to nivolumab. Cutaneous irAEs are a common side effect of ICIs, however, LyP secondary to ICIs is rare.
A systematic review of prospective trials evaluating the benefits and harms of nivolumab in patients with Hodgkin lymphoma after autoSCT identified 283 patients, with 12% reporting rash as an irAE, however, the rashes were not described [Citation12]. In general, skin reactions caused by ICIs include lichenoid reactions, eczema, vitiligo, and pruritus [Citation4]. There is one report of LyP secondary to an ICI in a patient with renal cell carcinoma [Citation4]. However, to our knowledge, there have been no reports of LyP associated with ICI in patients with Hodgkin lymphoma after autoSCT.
There are no prospective controlled studies validating treatments for LyP [Citation13]. However, current treatment methods include therapeutic abstention with supportive care for mild to moderate disease and oral, subcutaneous, or intramuscular methotrexate with weekly doses of 5 to 20 milligrams for moderate to severe disease [Citation13]. Other options include phototherapy, particularly psoralen and ultraviolet A (PUVA) therapy, and local chemotherapies including mechlorethamine, bexarotene, and interferon [Citation13]. For larger or recurrent LyP skin tumors, surgical excision or radiotherapy is another treatment option [Citation13]. Our patient, like the majority of patients with LyP, had resolution of disease with only supportive treatment, supporting the diagnosis of LyP rather than a malignant CD30+ disease.
Here we present a case of a 63-year-old male with recurrent Hodgkin lymphoma, treated with nivolumab and brentuximab followed by autoSCT, presenting with painful leg ulcers that on clinical and on pathological examination were consistent with a diagnosis of ulcerative LyP. To our knowledge, this is the first report of LyP potentially occurring as a reaction to immune checkpoint inhibitor-exposed T-lymphocytes from nivolumab therapy later activated by autoSCT. Physicians should consider ulcerative LyP in patients with Hodgkin lymphoma or with recent autoSCT receiving ICIs who develop painful ulcers. Clinically, these lesions may appear similar to cutaneous lymphoma or infection and biopsy is important to differentiate these entities.
Patient consent statement
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
Prior presentation
Contents of the manuscript have not been previously published and are not currently submitted elsewhere.
Reprint requests
Beth N McLellan, MD
| Abbreviations | ||
| ICI | = | immune checkpoint inhibitor |
| irAEs | = | immune-related adverse events |
| autoSCT | = | autologous stem cell transplant |
| LyP | = | lymphomatoid papulosis |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing not applicable – no new data generated
Additional information
Funding
References
- Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001.
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018 Jun;19(3):345–361. doi: 10.1007/s40257-017-0336-3.
- Toumi A, Fazal S, Litaiem N. Lymphomatoid papulosis. [Updated 2023 May 22]. statPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Ellis SR, Vierra AT, Millsop JW, et al. Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol. 2020;83(4):1130–1143. doi: 10.1016/j.jaad.2020.04.105.
- Collins LK, Chapman MS, Carter JB, et al. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017; 41(2):125–128. doi: 10.1016/j.currproblcancer.2016.12.001.
- Morita R, Kasai K. Lymphomatoid papulosis during immune checkpoint inhibitor treatment. Int J Hematol. 2022;116(2):155–157. doi: 10.1007/s12185-022-03410-z.
- Bush AE, Garcia A, Li J, et al. CD30+ lymphomatoid skin toxicity secondary to ipilimumab. JAAD Case Rep. 2020;6(4):251–253. doi: 10.1016/j.jdcr.2016.07.008.
- Moy A, Sun J, Ma S, et al. Lymphomatoid papulosis and other-lymphoma-like diseases. Dermatol Clin. 2019;37(4):471–482. doi: 10.1016/j.det.2019.05.005.
- de Leval L, Gaulard P. CD30+ lymphoproliferative disorders. Haematologica. 2010;95(10):1627–1630. doi: 10.3324/haematol.2010.029256.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34(1):59–73. doi: 10.1111/jdv.15931.
- Simonetta F, Pradier A, Bosshard C, et al. Dynamics of expression of programmed cell death protein-1 (PD-1) on T cells after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2019;10:1034. doi: 10.3389/fimmu.2019.01034.
- Roshandel E, Tavakoli F, Parkhideh S, et al. Post-hematopoietic stem cell transplantation relapse: role of checkpoint inhibitors. Health Sci Rep. 2022;5(2):e536. doi: 10.1002/hsr2.536.
- Goldkuhle M, Dimaki M, Gartlehner G, et al. Nivolumab for adults with Hodgkin’s lymphoma (a rapid review using the software RobotReviewer). Cochrane Database Syst Rev. 2018;7(7):CD012556.

