ABSTRACT
Kauri (Agathis australis), a tree species endemic to northern New Zealand, is threatened by kauri dieback disease, caused by the plant pathogen Phytophthora agathidicida. Some human and animal activities capable of disturbing and transferring infected soil may facilitate the spread of this pathogen. Feral pigs (Sus scrofa) are thought to be especially important due to their foraging behaviour. Consequently, management of feral pigs has been proposed as a key mitigation strategy for reducing the impacts of kauri dieback disease. We reviewed the evidence for feral pigs facilitating the spread of P. agathidicida. Although feral pigs can spread other plant pathogens, there is scant direct evidence that they act as vectors or transport hosts of P. agathidicida. Whether this is due to the lack of in-depth investigations, limitations in current detection methods, or simply that feral pigs are not playing a substantial role in the spread of kauri dieback disease is currently unknown. If management agencies proceed with a management strategy for feral pigs such as sustained control or eradication, under the assumption that this will mitigate unwanted impacts on kauri, an adaptive management approach should be taken to increase our understanding of the importance of feral pigs in this system.
Kauri dieback disease
Kauri, the only member of the family Araucariaceae in New Zealand, is an endemic conifer found in the northern part of the country. Once extensive, logging has substantially reduced the geographic extent of kauri forests. Nevertheless, kauri forests remain important specialised habitats for many species of native flora and faunae, and are important culturally (Jamieson et al. Citation2014; Hill et al. Citation2021). However, many of the remaining kauri forests are currently threatened by the plant pathogen Phytophthora agathidicida which can cause kauri dieback disease (Bradshaw et al. Citation2020).
Various species of soil-borne pathogens in the genus Phytophthora (which derives its name from Greek, meaning ‘plant destroyer’) can cause diseases in herbaceous and woody plants. Phytophthora species resemble fungi, but are oomycetes, a unique group of eukaryotic microorganisms. At least seven species of Phytophthora have been associated with kauri trees or soil in kauri forests within New Zealand, with two species reported to cause disease, P. cinnamomi and P. agathidicida (Beever et al. Citation2009; Weir et al. Citation2015; Manaaki Whenua – Landcare Research Citation2023). Phytophthora cinnamomi is present in many parts of the world, including Australia and Hawai‘i (Podger and Newhook Citation1971; Kliejunas and Ko Citation1976). It is found widely in kauri stands and can cause disease (Phytophthora root rot) but only occasional deaths of adult trees, particularly on poorly drained sites (Podger and Newhook Citation1971; Beever et al. Citation2009).
Phytophthora agathidicida (previously referred to as Phytophthora taxon Agathis, or PTA) can also cause disease in kauri, affecting the roots and lower trunk. This disease, known as kauri dieback disease, poses a threat to kauri at individual and population levels. Along with lesions to the roots and lower trunk, symptoms include excess resin production, the yellowing of foliage, and canopy thinning. New infections are difficult to detect, with obvious symptoms sometimes taking years to present. Infections usually lead to death of the tree, typically one to ten years after the development of symptoms (Bradshaw et al. Citation2020). First reported on Aotea/Great Barrier Island in 1972, P. agathidicida was subsequently confirmed on the mainland in 2006 and has since been confirmed in several locations within kauri range (Beever et al. Citation2009; Weir et al. Citation2015; Bradshaw et al. Citation2020). Links have also previously been made between plant nurseries and P. agathidicida spread (Beauchamp Citation2013).
The life cycle of P. agathidicida is complex, with multiple stages capable of dispersing within soil and free-flowing water. A plant host is initially infected by the zoospore stage, which is capable of actively swimming in surface water and soil water until encountering susceptible host material, which it is attracted to by chemical signals in the environment (Bradshaw et al. Citation2020). Although not tested under natural conditions, zoospores have been observed to survive and remain active for an average of 17 hours under laboratory conditions (Lawrence et al. Citation2017). In contrast, the more environmentally tolerant oospore stage, which can be found in infected host tissue (particularly roots) of both live specimens and decaying plant matter in the soil, can remain dormant for years under favourable conditions (Horner and Hough Citation2015; Bradshaw et al. Citation2020).
The various life cycle stages of P. agathidicida impact the survival and spread of the pathogen within the landscape in different ways. The zoospores are more important in local root-to-root transmission between neighbouring trees, whereas the oospores are more important to long-term survival and long-distance dispersal of the pathogen (Bradshaw et al. Citation2020). Therefore, the life cycle stage should always be considered in any P. agathidicida monitoring or mitigation efforts, as different approaches may need to be taken depending on the stage present.
Since some Phytophthora species can survive for extended periods outside of a host, particularly in soil, many activities that cause soil disturbance have been suggested as facilitating the spread of this pathogen (Podger and Newhook Citation1971; Kliejunas and Ko Citation1976; Bradshaw et al. Citation2020; Hill et al. Citation2021). For example, P. agathidicida and P. cinnamomi have been isolated from soil collected from hiking boots and boot cleaning stations, and from soil and debris from the exterior of vehicles (Kliejunas and Ko Citation1976; Hill et al. Citation2017, cited in Hill et al. Citation2021). Soil disturbance and movement by animals, both wild and domestic is, therefore, also of potential concern. For example, unfenced kauri fragments located among pastures are often subject to soil compaction by cattle (Bos taurus) (Waipara et al. Citation2013). Phytophthora agathidicida has also been isolated from a muddy cattle race (a narrow corridor along which cattle are moved, also called a cattle chute) located below an infected kauri stand (Beauchamp Citation2013).
Feral pigs (Sus scrofa) have been suggested as contributing to the spread of P. agathidicida, most notably via their foraging behaviour, which can disturb and transport significant amounts of soil potentially infected with P. agathidicida as well as their ability to physically damage trees, potentially making kauri more susceptible and/or less resilient to P. agathidicida. (Krull et al. Citation2013; Bassett et al. Citation2017; Bradshaw et al. Citation2020; Hill et al. Citation2021). Accordingly, management of feral pigs, pigs of domestic origin that have gone feral and now live in the wild, has been proposed as a way to mitigate this unwanted impact. Currently feral pigs are controlled in some parts of New Zealand with the goal of protecting biodiversity and/or production assets of value (Latham et al. Citation2020). In this article, we review and discuss the current evidence for feral pigs facilitating the spread of P. agathidicida and explore possible solutions for mitigating the damage that P. agathidicida causes to kauri forests, based on vertebrate pest management theory and adaptive management. Finally, we highlight key knowledge and research gaps that need to be addressed to optimise management aimed at protecting kauri forests. Our focus in this review is the ecology and management of feral pigs in the context of the asset, kauri, and the pathogen affecting kauri, P. agathidicida, rather than reviewing the broader impacts and management of feral pigs.
Ecology of feral pigs
Soil disturbance
Feral pigs are omnivorous and opportunistic, feeding on a variety of plant and animal food sources, many of which are associated with the soil layer. A study looking at the stomach contents of 104 feral pigs in Te Urewera observed over half (51.8%) of feral pigs’ annual diet was fruit and vertebrate carrion foraged on the ground (Thomson and Challies Citation1988). Plant roots and invertebrates obtained from below the surface were also a major source of food (30.6%). Browsing and grazing provided a smaller proportion of the overall diet (17.6%) in Te Urewera.
It is suspected that soil-associated feeding behaviours might be linked to the spread of P. agathidicida (Krull et al. Citation2013; Bassett et al. Citation2017). For example, ground rooting, often simply called ‘rooting’, is a common feeding behaviour in feral pigs whereby they dig or scrape into the soil with their snouts to obtain subterranean plant roots, bulbs, and invertebrates (VerCauteren et al. Citation2020). Feral pigs in New Zealand have been observed rooting in areas of P. agathidicida-infected kauri trees (Auckland Council Citation2011). Ground rooting can vary by season, with observations in Europe of more ground rooting by pigs occurring from late autumn to early summer, when above-ground sources of food were less abundant (Dardaillon Citation1987). This could result in a temporal effect in the role of pigs as vectors or transport hosts of P. agathidicida.
Ground foraging of fallen fruit might also be important because recent findings indicate that tawa trees (Beilschmiedia tawa) can also be infected by P. agathidicida (although this was only tested on roots and in a laboratory setting; Bradshaw et al. Citation2020), and the fruit of tawa can be a substantial component of the annual diet of feral pigs in some areas of New Zealand (21.4% of biomass by dry weight; Thomson and Challies Citation1988). Tawa occurs throughout the North Island and can form part of the lower canopy of kauri forests. In New Zealand, ground foraging of fruits by feral pigs often occurs seasonally, e.g. in Te Urewera, it was most common in autumn, followed by winter and summer (Thomson and Challies Citation1988).
Rooting behaviour by feral pigs is not limited to searching for plant material, but also includes consumption of below-ground fauna, usually invertebrates. One New Zealand study reported that earthworms comprise 10% of the annual biomass (by dry weight) of feral pig diet, with a frequency of 79% (82/104) of stomachs sampled containing earthworms (Thomson and Challies Citation1988). In a dietary study of 14 feral pigs in Fiordland, subterranean stag beetles (Geodorcus helmsi) made up 27% of the overall diet by dry weight, with earthworms and roots making up 0.7% and 5.8%, respectively (Parkes et al. Citation2015). The frequency of stag beetles, earthworms, and roots within stomachs was 86% (12/14), 29% (4/14), and 21% (3/14), respectively (Parkes et al. Citation2015). These below-ground dietary preferences highlight the close relationship feral pigs have with the soil environment.
Wallows are shallow depressions in the ground that are used and enlarged by pigs and other species (e.g. deer) for covering themselves in dirt or mud. Pigs exhibit wallowing behaviour primarily for thermoregulation, especially in warmer temperatures, but it is also done for protection against biting insects and ectoparasites and it may also be related to mating behaviour (VerCauteren et al. Citation2020). Since wallows are characterised by highly disturbed soil, in and adjacent to a depression which can promote standing water, they could be at higher risk of coming into contact with soil infected with P. agathidicida.
In addition to the potential transfer of soil from the snout (e.g. from rooting or ground foraging) or the body (e.g. from wallowing), a New Zealand study showed that feral pigs can carry up to 5 g of soil on each trotter (Krull et al. Citation2013). This study reported detection of P. cinnamomi from soil collected from a single trotter (out of 457 examined), although P. agathidicida was not detected. In Hawai‘i, P. cinnamomi was recovered from soil particles carried on the hooves of feral pigs (Kliejunas and Ko Citation1976), but a study in southwestern Australia was not able to detect P. cinnamomi from feral pig hooves (Li et al. Citation2014). The authors of this latter study attributed this discrepancy to the majority of soil having a high sand and gravel content and being readily dislodged when dry or from tree-rubbing activities. However, Li et al. (Citation2014) did find that P. cinnamomi remained viable within infected plant matter following passage through the digestive tract of feral pigs, highlighting another potential route of spread for Phytophthora species.
A study conducted in Waitutu Forest, Fiordland, reported 17.6% of the area surveyed showed some level of soil disturbance from feral pigs (Parkes et al. Citation2015). It was also observed that feral pigs were more likely to disturb sites they had previously disturbed than new sites. Similar observations have been made overseas, where Eurasian wild pigs (native to Eurasia and the wild counterpart of the feral pig) were seven times more likely to forage at previously disturbed sites than at new sites. It is not known if wild pigs reused these sites because of ease of access or due to them being prime foraging sites (Dardaillon Citation1987). It has been suggested that rooting by feral pigs can increase the presence of earthworms and ground beetles by altering soil conditions, helping to explain repeated rooting at the same sites (Wehr et al. Citation2020). If sites repeatedly visited by feral pigs coincide with areas of high risk of exposure to P. agathidicida, it could have implications for pathogen spread.
Wounding kauri trees
It has been suggested that pig damage to the roots or lower trunk of a kauri tree could indirectly contribute to kauri dieback disease, even if no pathogen is present at the time the damage occurs (Krull et al. Citation2013). The wound might provide a point of entry for a pathogen; this process is suspected to occur in Hawai‘i with rapid ‘ōhi‘a death (ROD), a fungal disease of native ‘ōhi‘a trees (Metrosideros polymorpha) (Mortenson et al. Citation2016; Perroy et al. Citation2021). Wounding can also add increased stress to the tree, which might influence susceptibility to infection. In addition to rooting, other damaging behaviours to trees by feral pigs include chewing on roots, girdling of the trunk, and damaging the bark by rubbing, tusking (i.e. marking scent using tusk glands), or browsing (VerCauteren et al. Citation2020). In the USA mainland feral pigs have been observed chewing on the roots of longleaf pine (Pinus palustris) seedlings and then consuming the sap and starches before spitting out the woody tissue (Wood and Roark Citation1980). Although it has been noted that feral pigs tend to prefer fleshy roots to woody roots, extensive rooting of the forest floor has been shown to cause damage to fine, woody root systems in Europe (Howe et al. Citation1981).
Feral pigs’ role in kauri dieback disease epidemiology
Theoretical mechanisms
Although there are some indications that feral pig presence may be correlated with kauri dieback disease distribution, there is a general lack of evidence, including no direct evidence, of how feral pigs might be contributing to the spread of P. agathidicida. However, if this is occurring, it could be through two different mechanisms (see ). The first mechanism involves facilitating pathogen spread through the transfer of infected soil or plant matter. This could lead to increases in incidence rates within an already infected area and/or increases in the total number of infected areas through spread to new, uninfected areas. These new infections could be the result of transmission of P. agathidicida directly to uninfected kauri trees (e.g. by chewing on tree roots) or through contamination of the local environment (e.g. via defecation, or the movement of infected soil). The second mechanism through which feral pigs could be contributing to kauri dieback may not directly involve the pathogen itself but may be related to changes in the susceptibility to infection of a potential kauri host (e.g. by causing wounds that act as entry points for pathogens, or increased stress to the tree). It is important to note that these mechanisms are theoretical, with investigations specific to both P. agathidicida and feral pigs necessary to better understand their likelihood – or even their possibility. To date, very few of these investigations have been carried out, with little substantive evidence implicating feral pigs in the spread of kauri dieback disease.
Figure 1. Hypothesised role of feral pigs (Sus scrofa) in the spread of kauri dieback disease in New Zealand. Arrows depict theoretical mechanisms by which various feral pig behaviours could contribute to Phytophthora agathidicida infections in kauri (Agathis australis). Note these are not established pathways, but only potential linkages, some of which could be tested through experimental studies.
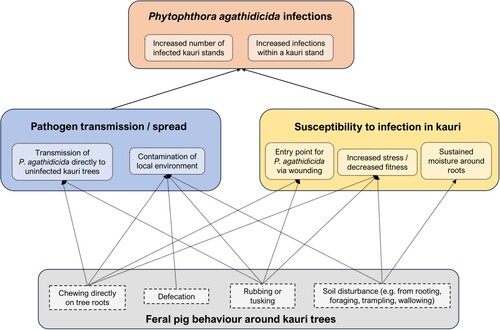
Current evidence
An early attempt was made to explore the relationship between feral pig presence and kauri dieback disease in Auckland Region (Auckland Council Citation2011). Alongside a ground-truthing survey of kauri dieback disease in the Waitākere Ranges, evidence of feral pig rooting was recorded and compared between kauri and non-kauri areas, and between the disease status of kauri trees (categorised as ‘infected’, ‘possibly infected’, and ‘healthy’). Results showed the likelihood of feral pig rooting within a kauri area to be 0.75%, and within a non-kauri area to be 0.03%. Although these observations indicate that foraging (i.e. rooting) by feral pigs was more prevalent in kauri areas, they also indicate that overall, irrespective of forest type, rooting was not common in the area at the time the survey was conducted. Results also showed that the likelihood of feral pig rooting around a healthy kauri tree was 0.44%, around a possible kauri dieback tree 0.12%, and around a known kauri dieback diseased tree 1.06%; however, it was not determined if these values differed statistically. Also, a positive relationship was reported between the proximity of feral pig activity and infected and possibly infected kauri trees. However, due to the limitations of the data collected, particularly since the rooting observations were made as part of a larger survey (e.g. no randomised sampling), these results are not conclusive, and should be treated as such, until a study that can determine causation can be conducted.
Krull et al. (Citation2013) investigated whether feral pigs were involved in the spread of Phytophthora pathogens within the Waitākere Ranges from 2008 to 2011. The results of swabs taken from 457 trotters (one trotter tested per animal) and 364 snouts showed one detection of P. cinnamomi from a single trotter, with no detections of P. agathidicida. The absence of positive samples was attributed to either low test sensitivities or uneven distribution of the pathogen in the environment. Despite P. agathidicida not being detected in this study, it was concluded, using a Bayesian probability model that there is a high probability of P. agathidicida being spread by feral pigs moving infected soil (Krull et al. Citation2013). An important limitation of Krull et al.’s (Citation2013) study was a lack of empirical data necessary to parameterise the probability model. To accommodate this, values for the design prevalence (i.e. the minimum number of pig snouts or trotters with P. agathidicida that could be detected with the sampling design used) were elicited from expert opinion and estimates range widely (0.05-0.35). Another influential parameter in the model, the prior probability that feral pigs carry P. agathidicida (i.e. what is the general belief before the study is conducted), was set extremely high at 0.9. This high value for the prior resulted in the model suggesting that a large sample of trotters (>1000) would be needed to be confident in a negative result such as the one the authors report. Thus, despite the lack of any positive detections, the authors concluded that there is a 35–90% chance that pigs may vector P. agathidicida. . Empirical data are needed to substantiate the conclusions of Krull et al. (Citation2013). However, as the authors state, appropriate and reliable tests capable of detecting P. agathidicida from small soil samples associated with animal tissue are needed before continuing to investigate the transmission potential of pigs and other possible candidates.
Bassett et al. (Citation2017) investigated the potential for feral pigs to spread P. agathidicida via passage through the digestive tract. The study included hunter-collected feral pig stomach samples from the Waitākere Ranges, as well as a captive feeding experiment with domestic pigs (S. scrofa domesticus) using kauri roots infected with P. agathidicida. Of the 184 hunter-collected stomach samples, 23 (12.5%) tested positive for Phytophthora spp. These positive samples belonged to five species, including P. cinnamomi (5/23), but no P. agathidicida was detected. Results from the captive feeding experiment, which included infected kauri root material and millet seeds passed through the gut passage of 12 pigs, included a single detection of P. agathidicida from a kauri root found in pig faeces. The study also inoculated kauri seedlings with faeces produced during the feeding trial, with no infections of the seedlings observed. The results of this study showed that P. agathidicida is capable of remaining viable following ingestion and passage through the gut of a pig. This single observation is the first and only proof-of-concept evidence for the potential of feral pigs acting as vectors or transport hosts of P. agathidicida. Based on their findings, the authors suggested that the risk of P. agathidicida spread through the gut passage of feral pigs may be considerably lower than that of P. cinnamomi. They hypothesised that P. agathidicida may be less robust than P. cinnamomi in terms of the temperatures encountered in the digestive process of feral pigs. Due to the low incidence of P. agathidicida detected in feral pigs in this study, the authors recommended prioritising the management of human-mediated soil movement when attempting to reduce the risk of kauri dieback disease spread. However, they also recommended that when taking a precautionary approach to forest management, feral pigs should be considered at least a minor transmission pathway for P. agathidicida but should still be considered a major transmission pathway for other Phytophthora species, particularly P. cinnamomi.
Next steps for epidemiological research
Overall, there is scant evidence that feral pigs are acting as vectors or transport hosts of P. agathidicida in New Zealand. Whether this is due to the lack of overall investigations, limitations in current detection methods, or simply that feral pigs (or ungulates in general) are not playing a substantial role in the spread of kauri dieback disease is currently unknown. Further research is necessary to tease apart these possible explanations ().
Table 1. Summary of research needs for investigating the role of feral pigs (Sus scrofa) in the spread of Phytophthora agathidicida, the causative agent of kauri dieback disease in New Zealand.
A potential first step could be to investigate any correlations that may exist between feral pigs and kauri dieback disease. One option for this would be to re-evaluate the relationships explored by Auckland Council (Citation2011), which compared feral pig presence and kauri dieback disease status. Another option would be to adapt research approaches being used for other forest – disease systems. For example, in Hawai‘i, a monitoring study was used to compare ungulate activity (which included feral pigs, goats, (Capra hircus), sheep (Ovies aries), mouflon (O. gmelini musimon), and cattle) with ROD-associated tree mortality (Perroy et al. Citation2021). Ultimately, it was shown that ungulate presence, determined by fenced (i.e. ungulate-free) and unfenced (i.e. ungulate present) areas, strongly affected spatial patterns of ROD.
If a correlation was found, subsequent studies may look for causation and be mechanistic to gain a more thorough understanding of the epidemiological role of pigs. Only once it was determined that feral pigs were acting as key vectors or transport hosts of P. agathidicida, would the next step be to understand how much they contribute to pathogen spread relative to other biotic and abiotic mechanisms, including human-mediated soil movement. The prevalence of P. agathidicida within the feral pig population(s) would also need to determined, in and adjacent to kauri forests, and if prevalence varied spatially or temporally. The lack of such information makes it very challenging to determine the appropriate strategic, tactical, and logistical approaches for managing feral pigs to achieve the desired outcome of reducing the spread of P. agathidicida. Also, the recent finding that P. agathidicida can infect and colonise other native plants associated with kauri (such as tawa, a food source for feral pigs) adds a further layer of complexity to any potential surveillance and management programme (Bradshaw et al. Citation2020).
Feral pig management
Management strategies
While managers could proceed with sustained control or eradication of feral pig populations under the assumption that these strategies will mitigate the unwanted impacts feral pigs are thought to be having on kauri, such a precautionary approach would be based on scant evidence. Without sound ecological and epidemiological information to guide strategies, tactics and logistics, the outcomes defined in pest management plans are unlikely to be met. However, if robust data are collected during such operations, these data may form a useful part of an adaptive management plan (see ‘Adaptive management’ below) for feral pigs and kauri. Importantly, since feral pigs are considered a valued resource by some stakeholders, managers must be able to justify and defend the management of this pest species.
Four strategic options are available for managing the unwanted impacts caused by invasive vertebrate pests. The first two are positive strategic options for managing a pest that is already present in an area. The third option is preventive and is the strategy of key importance if the pest has not yet spread to an area. The final option is neither preventive nor positive for the asset(s) being protected.
If feral pigs are present, one strategic option is sustained management (e.g. sustained lethal control of the pest, or fencing out the pest to protect the asset). This management action may have to be sustained in perpetuity to achieve a positive outcome. However, given the high financial expenditures associated with managing pests in perpetuity, option 2, eradication (see below), is increasingly favoured as a better long-term strategy for delivering high ecological benefits (e.g. Russell et al. Citation2015).
A second option in areas with feral pigs and kauri is eradication. This management action will provide positive outcomes for species or ecosystems threatened by feral pigs if eradication can be achieved. However, due to the uncertain role feral pigs play in the spread of kauri dieback disease, eradication of pigs may not be beneficial for kauri. While eradication is often a favoured strategy, it is challenging and makes policy sense only if it is achievable (Parkes and Murphy Citation2003; Parkes et al. Citation2017). For eradication to be achievable several criteria must be met. Critically, the rate of removal must exceed the rate of increase at all population densities; immigration must be prevented; and all reproductive animals must be put at risk (Parkes Citation1990). Additionally, all target animals must be detectable at low densities; a discounted cost – benefit analysis should favour eradication over sustained control (especially relevant for production assets); and the social, political, and economic environment must be suitable for attempting eradication (Bomford and O’Brien Citation1995; Parkes and Panetta Citation2009). Before proceeding with eradication as a strategy, a feasibility plan should be done to determine if eradication is achievable and if it is likely to produce the desired outcomes.
In areas with no feral pigs (or P. agathidicida), the best management action may be to stop the pest arriving in the first place. For example, it may be beneficial to establish a sustained control programme for pigs in a buffer zone to prevent their range expansion into adjacent areas containing uninfected kauri. Note: once a tree becomes infected with P. agathidicida, it is thought to remain infected, so preventing pathogen spread to new areas is a priority.
A fourth option is to do nothing. While we do not advocate doing nothing, it may be unavoidable, at least in some areas, because of high management costs. As additional ecological and epidemiological information are collected, priorities may change and areas not initially identified for management, may be subsequently identified as important.
Strategies, tactics, and tools have recently been reviewed for managing feral pigs in the northern North Island, with recommendations for ongoing sustained control to low densities for feral pig populations in or near important kauri forests (i.e. the precautionary approach, discussed above, was applied) (Latham and Yockney Citation2020). However, the authors noted that a threshold of acceptable feral pig density cannot currently be defined due to a lack of ecological and epidemiological data. Similarly, they cautioned against the broadscale use of fencing, but suggested it could be useful for protecting uninfected kauri forests in some areas if feral pigs and P. agathidicida are not already present. This feral pig-focused fencing strategy does not consider the role that other mechanisms of pathogen spread may be playing, nor the relative contributions each may be having on pathogen transmission. For example, if flowing water, humans, or other ungulates have access to the fenced area, and if they commonly spread the pathogen, then a management strategy focused on excluding feral pigs would probably not be effective for preventing movement of the pathogen.
Latham and Yockney (Citation2020) also proposed a suite of control tools that have known or potential efficacy in reducing feral pig densities. Key among these were professional ground and aerial hunting (including using thermal imaging technology) and trapping. They also list potentially useful toxins, such as sodium nitrite, and rank their potential for controlling feral pigs in New Zealand (see Appendix 1). However, since the level of feral pig reduction required to mitigate impacts to kauri is currently unknown, identifying appropriate control tools – or at least ruling out certain approaches – may not be possible at this stage. For example, while recreational hunting may be useful for controlling feral pigs in production landscapes (e.g. shooting a mob of feral pigs on a fodder beet crop), it would probably not be an appropriate control method for protecting kauri, based on evidence showing that recreational hunting rarely reduces the density of pests to low enough population levels to mitigate certain unwanted impacts (Bengsen et al. Citation2020). Moreover, managing feral pigs to mitigate their impacts in kauri forests will require a robust and well-articulated adaptive management programme (see discussion directly below). Adaptive management requires robust data collection, and recreational hunters should not be depended on to collect such data.
Adaptive management
Uncertainty is a pervasive feature of many pest management programmes. Lethal control kills pests, often in large numbers. However, the percentage by which the pest population is reduced, the residual number of pests, the threshold of pest numbers necessary to protect the asset(s) of concern, and the response of the asset(s) to pest control are often unknown, or poorly understood. Eradication programmes face similar uncertainties, notably knowing the geographical extent of the pest population and proving that an attempt to eradicate the pest population was indeed successful. These problems can result in ineffective pest management programmes.
A potential solution to these uncertainties is adaptive management (Holling Citation1978; Walters Citation1986). Adaptive management emphasises the identification of critical ecological uncertainties and the design of diagnostic management experiments to reduce these uncertainties (Walters Citation2011). The term ‘adaptive management’ is often tacked on to a management programme and used as justification to proceed with poorly planned management, often in contexts where adaptive management is inappropriate and its application not feasible (Rist et al. Citation2013). In many cases this has resulted in adaptive management failing to increase our knowledge about the ecological uncertainties within the system of interest.
For adaptive management to be appropriate and useful, ecological uncertainty, a key obstacle, should be reduced experimentally and through carefully designed system monitoring. Clearly, there is ecological uncertainty in relation to managing feral pigs to mitigate their impacts on kauri, and monitoring programmes with sound data collection will help to clarify a number of important knowledge gaps and research needs (). For example, it is critical to confirm (or otherwise) feral pigs as a major vector or transport host of P. agathidicida and, if they are, to estimate the proportion of the feral pig population that is playing a role in the spread of the pathogen, which will probably vary temporally and spatially. Subsequently, it will need to be determined if a high prevalence of P. agathidicida in a feral pig population is because feral pigs are responsible for the introduction of the pathogen to an area, or because they simply picked up the pathogen from soil that was already heavily infected with P. agathidicida, possibly introduced through a different mechanism, such as water transmission or another animal species.
In addition to understanding the role of feral pigs in kauri dieback disease, epidemiologists will need to determine what outcome(s) (e.g. reduction in the prevalence of P. agathidicida in the feral pig populations, reduction in total number of individuals, reductions at certain times of the year) should be assessed for determining if feral pig management has mitigated the unwanted impacts. Samples collected as part of a sustained control or eradication programme may be able to help answer these questions. Although not constituting experimental adaptive management, this system monitoring has the potential to reduce some uncertainty.
A critical consideration for managers is determining which strategies or aspects of feral pig management may benefit most from experimental adaptive management. First, it is worth reiterating that managers will likely apply the precautionary principle when managing feral pigs to mitigate their hypothesised impacts on kauri. There are a number of management strategies for feral pigs that will probably be applied across kauri habitat; e.g. prevent feral pigs spreading to (or being released in) areas that currently do not have feral pigs, institute sustained control, fence uninfected kauri forests without feral pig populations, localised feral pig eradication, and doing nothing. We do not discuss ‘doing nothing’ here, though in reality it may be unavoidable in some areas.
Eradication is a strategy that permanently removes the unwanted impacts caused by the pest, assuming that all individuals can be removed and reinvasion is preventable. Importantly, however, if feral pigs are not a key vector or transport host, eradicating them will not achieve positive outcomes for kauri, though it will do for other indigenous, or production assets impacted by pigs. Key uncertainties for an eradication strategy include delineating the target population and proving eradication at the conclusion of a management intervention. Determining detection probabilities and surveillance sensitivities for use in proof-of-eradication models will be necessary for addressing these uncertainties (Anderson et al Citation2013). Sustained control programmes that aim to prevent feral pigs spreading to new areas will also benefit from this surveillance methodology.
Eradication may not be achievable everywhere over the kauri geographic range, due to various logistical impediments as well as lack of widespread social buy-in by many stakeholders. Therefore, sustained control and/or fencing may be necessary to protect kauri forests in some locations. We suggest that experimental adaptive management may be most beneficial for elucidating uncertainties related to sustained control and fencing. For example, patches of kauri forest without feral pig populations (or no or low prevalence of P. agathidicida in the trees or soil) could be fenced to prevent feral pig invasion. Some measure of kauri health or P. agathidicida presence could be undertaken annually and compared with similar data from nearby unfenced kauri forests that have been recently invaded by feral pigs. Fences that are capable of excluding feral pigs are likely to be expensive and will require continual maintenance, especially where tree falls are likely to damage fences. For these reasons they are unlikely to be a suitable broadscale strategy for managing feral pig movements. Nevertheless, they have proven effective in some scenarios, e.g. in Hawai‘i (Perroy et al. Citation2021) and Northland, New Zealand (Aviss and Roberts Citation1994), and may be useful for preventing feral pigs and other potential vectors or transport hosts of P. agathidicida from accessing uninfected kauri forests if the terrain and vegetation are suitable.
If feral pigs play an important role in pathogen spread or severity of disease, the prediction would be that fenced forests would have lower prevalence of P. agathidicida in the soil than unfenced forests. Although only correlational (as seen with the Perroy et al. Citation2021 Hawai‘i ROD study), this would, in the absence of data confirming feral pigs as vectors or transport hosts of P. agathidicida, be reasonably compelling evidence that they play an important role, especially if there were multiple replicates of fenced and unfenced forests that showed the same pattern. Importantly, however, other factors could play a confounding role in data interpretation, such as the presence of other potential mechanisms of pathogen spread (e.g. people hiking in the forests, the presence of goat populations, water moving P. agathidicida into fenced areas, high natural variability between areas). Therefore, additional data should be collected to tease apart which factor(s) might be most important.
In investigating ROD in Hawai‘i, high-resolution aerial imagery was used to compare canopy condition between areas with and without ungulates present. It was noted that one challenge presented by using these aerial mapping methods was the need for ground-sampling of suspect trees to confirm the cause of tree mortality. Although results of this study showed a correlation between ungulate presence and ROD-associated tree mortality, the study did not seek to determine the specific mechanisms involved, something that additional targeted research would be needed to determine. In the meantime, besides possibly tree felling (which can include covering infected trees with tarps and/or spraying with insecticide to discourage wood-boring insect vectors), excluding ungulates from forested areas seems to be one of the only ROD management practices available for land managers in Hawai‘i (Perroy et al. Citation2021).
Adaptive management for a sustained feral pig control programme could be similarly designed, although ideally it would be multifactorial. That is, we are interested in understanding the relationship between pest density (or abundance, or relative abundance) and the asset (i.e. prevalence of P. agathidicida in kauri forests), known as a density – impact function (DIF). However, it is difficult to determine a DIF directly for feral pigs and P. agathidicida infection in kauri forests because it has not yet been confirmed that feral pigs are indeed acting as vectors or transport hosts within the landscape. Therefore, it is not currently possible to understand how infection levels change across a range of feral pig densities, or what effect seasonal or annual variation has on this relationship.
Nevertheless, using a multi-factorial, replicated design that includes controlling feral pigs to moderate numbers in some areas versus low numbers in others, and then determining how the asset responds spatially, seasonally and across years, may be one way of adaptively learning about ecological uncertainties in the system. Again, this would not be direct evidence that feral pigs are involved, but if incidence levels or rates of spread decreased following intensive reductions in feral pig numbers, it would be strong indirect evidence that feral pigs are playing an important role. This methodological design would be further strengthened (without providing cause and effect) by subsequently reducing feral pig numbers in the treatment blocks that have received little or no sustained control to low levels and determining how the asset responds.
It is important to note that adaptive management is just one management tool, and that it is always embedded in a larger management framework that involves social, political, and institutional elements. Decisions about the implementation of adaptive management must recognise these broader considerations. This is likely to be especially important for large, multi-factorial adaptive management experimentation, such as a sustained control strategy, because this will be costly and affect a wide range of stakeholders, across a large geographical extent.
Conclusions
In summary, without strong evidence about the role feral pigs play in the spread of P. agathidicida, and knowledge about the level of feral pig control necessary to achieve positive outcomes for kauri, managers are largely ‘flying blind’. If feral pigs are confirmed as vectors or transport hosts, or aid in transmission/infection through other means (such as open injuries to kauri roots), information will be needed to determine the appropriate densities feral pigs must be reduced to for mitigating their role in spreading P. agathidicida. Ideally, this would be done by estimating seasonal density – impact functions (DIFs).
However, given the complexity of this system, getting the data to construct robust DIFs to guide management will be challenging. For example, there will probably be lag effects and significant inherent variability in the system. To estimate a DIF, assessments must be done at multiple areas with different feral pig densities. In this instance, the DIF will probably be confounded by the fact that the prevalence of P. agathidicida will vary across feral pig populations of similar densities. This will mean it will not be possible to establish a threshold of feral pig density to mitigate the spread of P. agathidicida. However, these complexities should not be an excuse for not trying to increase our understanding of the relationship between the density of the pest and the impact on the asset.
Increasing our understanding of the relationship between the pest and the asset must be done in conjunction with obtaining a greater epidemiological understanding of other mechanisms involved, especially the relative magnitude that each may be playing in the spread of P. agathidicida. If other modes of transmission (e.g. flowing water, human activity, other animal vector or transport hosts) are themselves responsible for unacceptable levels of pathogen spread, then a management strategy specifically focused on feral pigs may not be appropriate, in terms of both effort and disease prevention itself.
Ecological and epidemiological data are needed for effective management plans. In the meantime, ongoing sustained control of feral pigs in the areas of greatest concern, or fences around areas that are known to be free of both feral pigs and P. agathidicida, may prevent the spread of P. agathidicida. However, they may not, and any outcome would need to be measured. Ideally, adaptive management – perhaps using before-after-control-impact (BACI) study designs, including treatment and control sites – is needed to explicitly explore the effectiveness of each strategy; otherwise, these efforts are implemented blindly, and will not generate new information that is robust and comparable, ultimately leaving their effectiveness impossible to gauge.
We acknowledge that population monitoring will form an important part of any feral pig management programme that aims to mitigate their unwanted impacts on kauri. While there are no well-established or consistently used monitoring techniques in New Zealand for measuring feral pig abundance (actual or relative), a variety of techniques have been utilised elsewhere (NPCA Citation2018). Some of these may be specifically useful in New Zealand, such as daytime inspections for field sign or soil disturbance, faecal counts, and catch-per-unit effort. However, before a monitoring programme can be designed, the role of feral pigs in the system will need to be better understood, especially determining what density feral pig populations need to be reduced to in order to mitigate their impacts on kauri. In the interim, a general assessment of the efficacy of potential survey methods (such as aerial thermal imagery and camera traps, and related power analyses) may be worthwhile. It is also important to consider the appropriateness of various indices, such as relative abundance, when assessing the effects of management efforts, especially when making spatial or temporal comparisons.
The tactics used in any sustained control or eradication operations should be guided by feral pig ecology (e.g. home range sizes, movement ecology, dispersal) in the northern North Island. Eradication of some feral pig populations may be feasible and justifiable. However, extreme policies risk losing buy-in from important stakeholders (e.g. those communities that value feral pigs for hunting and as a source of food) and should not proceed without robust data demonstrating the role of feral pigs, or feasibility assessments demonstrating that eradication is a viable strategy. Further research is needed to address the key knowledge gaps relating to the role of feral pigs in the kauri dieback disease system in New Zealand.
Acknowledgements
We thank Dean Clarke, Tony Beauchamp, other members of the New Zealand Department of Conservation, M. Cecilia Latham, and Andrew Gormley for their feedback on earlier drafts and particular subject material inquiries.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anderson D, Ramsey D, Nugent G, Bosson M, Livingstone P, Martin P, Sergeant E, Gormley A, Warburton B. 2013. A novel approach to assess the probability of disease eradication from a wild-animal reservoir host. Epidemiology and Infection. 141:1509–1521. doi:10.1017/S095026881200310X.
- Auckland Council. 2011. An investigation into the distribution and spread of kauri dieback disease within the Waitakere Ranges. Draft Report. 2011. Auckland Council. Auckland, New Zealand.
- Aviss M, Roberts A. 1994. Pest fences: notes and comments. Threatened Species Occasional Publication No. 5. Department of Conservation. Wellington, New Zealand.
- Bassett IE, Horner IJ, Hough EG, Wolber FM, Egeter B, Stanley MC, Krull CR. 2017. Ingestion of infected roots by feral pigs provides a minor vector pathway for kauri dieback disease Phytophthora agathidicida. Forestry: An International Journal of Forest Research. 90:640–648. doi:10.1093/forestry/cpx019.
- Beauchamp AJ. 2013. The detection of Phytophthora taxon “Agathis” in the second round of surveillance sampling – with discussion of the implications for kauri dieback management of all surveillance activity. Unpublished Report.
- Beever R, Waipara M, Ramsfield T, Dick M, Horner I. 2009. Kauri (Agathis australis) under threat from Phytophthora? Proceedings of the Fourth Meeting of the International Union of Forest Research Organizations (IUFRO) Working Party; August 26–31, 2007; Monterey, California, USA. p. 74–85.
- Bengsen AJ, Forsyth DM, Harris S, Latham ADM, McLeod SR, Pople A. 2020. A systematic review of ground-based shooting to control overabundant mammal populations. Wildlife Research. 47:197–207. doi:10.1071/WR19129.
- Bomford M, O’Brien P. 1995. Eradication or control for vertebrate pests? Wildlife Society Bulletin. 23:249–255.
- Bradshaw RE, Bellgard SE, Black A, Burns BR, Gerth ML, McDougal RL, Scott PM, Waipara NW, Weir BS, Williams NM, et al. 2020. Phytophthora agathidicida: research progress, cultural perspectives and knowledge gaps in the control and management of kauri dieback in New Zealand. Plant Pathology. 69:3–16. doi:10.1111/ppa.13104.
- Dardaillon M. 1987. Seasonal feeding habits of the wild boar in a Mediterranean wetland, the Camargue (Southern France). Acta Theriologica. 32:389–401. doi:10.4098/AT.arch.87-27.
- Hill L, Ashby E, Waipara N, Taua-Gordon R, Gordon A, Hjelm F, Bellgard SE, Bodley E, Jesson LK. 2021. Cross-cultural leadership enables collaborative approaches to management of kauri dieback in Aotearoa New Zealand. Forests. 12:1671–1617. doi:10.3390/f12121671.
- Hill L, Waipara N, Horner I. 2017. Ongoing monitoring of kauri dieback risk with Auckland Councils Regional Park network. Unpublished Report.
- Holling CS. 1978. Adaptive environmental assessment and management. Chichester: UK, John Wiley and Sons.
- Horner I, Hough E. 2015. Assay of stored soils for presence of Phytophthora agathidicida. A plant and food research report prepared for the ministry for primary industries. Contract No. 32294. Auckland, New Zealand.
- Howe TD, Singer FJ, Ackerman BB. 1981. Forage relationships of European wild boar invading northern hardwood forest. The Journal of Wildlife Management. 45:748–754. doi:10.2307/3808713.
- Jamieson A, Bassett IE, Hill LMW, Hill S, Davis A, Waipara NW, Hough EG, Horner IJ. 2014. Aerial surveillance to detect kauri dieback in New Zealand. New Zealand Plant Protection. 67:60–65. doi:10.30843/nzpp.2014.67.5723.
- Kliejunas JT, Ko W. 1976. Dispersal of Phytophthora cinnamomi on the Island of Hawaii. Phytopathology. 66:457–460.
- Krull CR, Waipara NW, Choquenot D, Burns BR, Gormley AM, Stanley MC. 2013. Absence of evidence is not evidence of absence: Feral pigs as vectors of soil-borne pathogens. Austral Ecology. 38:534–542. doi:10.1111/j.1442-9993.2012.02444.x.
- Latham ADM, Latham MC, Norbury GL, Forsyth DM, Warburton B. 2020. A review of the damage caused by invasive wild mammalian herbivores to primary production in New Zealand. New Zealand Journal of Zoology. 47:20–52. doi:10.1080/03014223.2019.1689147.
- Latham ADM, Yockney I. 2020. A review of control strategies and tools for feral pigs. Manaaki Whenua – Landcare Research Contract Report LC3699 for Northland Regional Council.
- Lawrence SA, Armstrong CB, Patrick WM, Gerth ML. 2017. High-throughput chemical screening identifies compounds that inhibit different stages of the Phytophthora agathidicida and Phytophthora cinnamomi life cycles. Frontiers in Microbiology. 8:1–10. doi:10.3389/fmicb.2017.01340.
- Li AY, Williams N, Fenwick SG, Hardy GESJ, Adams PJ. 2014. Potential for dissemination of Phytophthora cinnamomi by feral pigs via ingestion of infected plant material. Biological Invasions. 16:765–774. doi:10.1007/s10530-013-0535-7.
- Manaaki Whenua – Landcare Research. 2023. New Zealand fungi names databases – agathis australis (D.Don) lindl. Association Data (https://BiotaNZ.landcareresearch.co.nz/scientific-names/40967c73-3c4a-41a2-8025-5da89fba94a8/associations) (accessed 09 November 2023).
- Mortenson LA, Hughes RF, Friday JB, Keith LM, Barbosa JM, Friday NJ, Liu Z, Sowards TG. 2016. Assessing spatial distribution, stand impacts and rate of Ceratocystis fimbriata induced ‘ōhi‘a (Metrosideros polymorpha) mortality in a tropical wet forest, Hawai‘i Island, USA. Forest Ecology and Management. 377:83–92. doi:10.1016/j.foreco.2016.06.026.
- NPCA. 2018. Feral pigs: a review of monitoring and control techniques. Wellington, New Zealand: National Pest Control Agencies.
- Parkes J. 1990. Eradication of feral goats on islands and habitat islands. Journal of the Royal Society of New Zealand. 20:297–304. doi:10.1080/03036758.1990.10416824.
- Parkes J, Murphy E. 2003. Management of introduced mammals in New Zealand. New Zealand Journal of Zoology. 30:335–359. doi:10.1080/03014223.2003.9518346.
- Parkes J, Panetta FD. 2009. Eradication of invasive species: progress and emerging issues in the 21st century. In: Clout MN, Williams PA, editor. Invasive species management: a handbook of principles and techniques. Oxford: Oxford University Press; p. 47–60.
- Parkes JP, Easdale TA, Williamson WM, Forsyth DM. 2015. Causes and consequences of ground disturbance by feral pigs (Sus scrofa) in a lowland New Zealand conifer–angiosperm forest. New Zealand Journal of Ecology. 39:34–42.
- Parkes JP, Nugent G, Forsyth DM, Byrom AE, Pech RP, Warburton B, Choquenot D. 2017. Past, present and two potential futures for managing New Zealand’s mammalian pests. New Zealand Journal of Ecology. 41:151–161.
- Perroy RL, Sullivan T, Benitez D, Hughes RF, Keith LM, Brill E, Kissinger K, Duda D. 2021. Spatial patterns of ‘Ōhi‘a mortality associated with rapid ‘Ōhi‘a death and ungulate presence. Forests. 12:1035–1017. doi:10.3390/f12081035.
- Podger FD, Newhook FJ. 1971. Phytophthora cinnamomi in indigenous plant communities in New Zealand. New Zealand Journal of Botany. 9:625–638. doi:10.1080/0028825X.1971.10430225.
- Rist L, Felton A, Samuelsson L, Sandström C, Rosvall O. 2013. A new paradigm for adaptive management. Ecology and Society. 18:63. doi:10.5751/ES-06183-180463.
- Russell JC, Innes JG, Brown PH, Byrom AE. 2015. Predator-free New Zealand: conservation country. BioScience. 65:520–525. doi:10.1093/biosci/biv012.
- Thomson C, Challies CN. 1988. Diet of feral pigs in the podocarp-tawa forests of the Urewera ranges. New Zealand Journal of Ecology. 11:73–78.
- VerCauteren KC, Beasley JC, Ditchkoff SS, Mayer JJ, Roloff GJ, Strickland BK. 2020. Invasive wild pigs in North America: ecology, impacts, and management. Boca Raton, Florida, USA: CRC Press.
- Waipara NW, Hill S, Hill LMW, Hough EG, Horner IJ. 2013. Surveillance methods to determine tree health, distribution of kauri dieback disease and associated pathogens. New Zealand Plant Protection. 66:235–241. doi:10.30843/nzpp.2013.66.5671.
- Walters CJ. 1986. Adaptive management of renewable resources. New York, USA: Macmillan.
- Walters CJ. 2011. Is adaptive management helping to solve fisheries problems? AMBIO: A Journal of the Human Environment. 36:304–307. doi:10.1579/0044-7447(2007)36[304:IAMHTS]2.0.CO;2.
- Wehr NH, Litton CM, Lincoln NK, Hess SC. 2020. Relationships between soil macroinvertebrates and nonnative feral pigs (Sus scrofa) in Hawaiian tropical montane wet forests. Biological Invasions. 22:577–586. doi:10.1007/s10530-019-02117-3.
- Weir BS, Paderes EP, Anand N, Uchida JY, Pennycook SR, Bellgard SE, Beever RE. 2015. A taxonomic revision of Phytophthora clade 5 including two new species, Phytophthora agathidicida and P. cocois. Phytotaxa. 205:21–38. doi:10.11646/phytotaxa.205.1.2.
- Wood GW, Roark DN. 1980. Food habits of feral hogs in coastal South Carolina. The Journal of Wildlife Management. 44:506. doi:10.2307/3807990.
Appendix 1.
Table listing advantages and disadvantages of prospective vertebrate toxic agents for control of feral pigs (Sus scrofa) in New Zealand (reproduced from Latham & Yockney Citation2020). Only one bait, Bait-Rite Paste® (containing the sodium nitrite), is currently registered for feral pig control in New Zealand. Potential use in New Zealand is ranked assuming registration is readily achievable; if it is not, all options except Bait-Rite Paste® have low potential for use in New Zealand.

