?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Apigenin and gallic acid are natural compounds that are useful as antioxidant, anti-inflammatory and anticancer agents, especially when used together in combination. Therefore, the development and validation of a simultaneous method of analysis for both compounds in pure form and when encapsulated in an advanced delivery system such as liposomes would be useful.
Methods
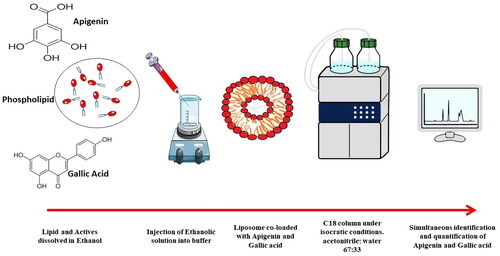
Analysis was performed using C18 column under isocratic conditions. The mobile phase was acetonitrile: water containing 0.2% orthophosphoric acid at a ratio of 67:33, flow rate 1 ml/min, and detection wavelength 334 nm for apigenin and 271 nm for gallic acid.
Results
The assay method was linear at the concentration range (5–600 µg/mL) with R2 of 1 for both drugs. The method was also shown to be precise and robust with RSD less than 2% with LOD (0.12, 0.1 µg/mL) and LOQ (4.14, 3.58 µg/mL) for apigenin and gallic acid respectively. The method was also applicable for the determination of the entrapment efficiency of both drugs when co-loaded in a nanoliposomal formulation.
Conclusion
The described HPLC method was shown to be suitable, sensitive, and reproducible for the simultaneous identification and quantification of apigenin and gallic acid. The analytical results were accurate and precise, with good recovery, low limit of detection, and the chromatographic assay was accomplished in less than 3 min, suggesting the suitability of the method for routine analysis of both drugs in pharmaceutical formulations.
Introduction
Apigenin is a naturally occurring flavonoid (5,7-Trihydroxyflavone) found abundantly in many fruits and vegetables (). It exhibits several biological and pharmacological activities such as anti-inflammatory, antioxidant, antiviral, and anticancer [Citation1–3]. Apigenin was recently used as a dietary supplement for cancer patients, and several researchers have encapsulated it in many dosage forms for its promising anticancer activity in inhibiting tubulin binding, induction of apoptosis and autophagy, and modulation of the cell cycle [Citation2,Citation4–6]. Apigenin has a molecular weight of 270.05 g/mol and log P of 3.1. It is poorly soluble in water and soluble in ethanol and dimethyl sulfoxide (DMSO), therefore it is classified as a class two drug according to the Biopharmaceutical Classification System (BCS) [Citation7]. Therefore, the encapsulation of apigenin in lipidic self-assembled systems is expected to efficiently solubilize it, for enhanced and sustained therapeutic activity.
Gallic acid () is another phenolic acid-based nutraceutical found in several sources, especially grapes and tea. It exhibits many pharmacological activities as a powerful antioxidant, anticancer, anti-inflammatory, and antibacterial. It is soluble in most solvents (water, ethanol, and methanol) with a molecular weight of 170.12 g/mol [Citation8,Citation9]. Despite its complete oral absorption, gallic acid would benefit from encapsulation in lipidic self-assembled systems to enhance its therapeutic activity and sustain its release [Citation10].
Several reports in the literature have proven that combining two nutraceuticals in one dosage form or a delivery system mostly maximize their therapeutic efficacy [Citation11,Citation12]. In particular, co-loading of nutraceuticals alone or in combination with chemotherapeutic agents have been proven efficacious in the treatment of many cancer types [Citation13–17]. Therefore, the combination of apigenin and gallic acid in one delivery system is expected to synergize their anticancer activity by different mechanistic pathways, such as induction of apoptosis and autophagy, inhibition of proliferation, inhibition of cancer cell migration and invasion, cell cycle arrest, and increased reactive oxygen species [Citation3,Citation18].
Among the promising delivery systems reported till current date is liposomes, which is a self-assembled lipidic system based on phospholipids, effectively known to load both water soluble and insoluble drugs [Citation19–24], and therefore, it would be an optimum system for co-loading of the water insoluble apigenin, and the water soluble gallic acid. In addition, when either apigenin or gallic acid was loaded in liposomes, an increased cellular uptake and cytotoxic activity was reported compared to their free forms [Citation5,Citation18,Citation25–27].
From an analytical perspective, several methods have been developed for the determination of apigenin and gallic acid alone, with the disadvantage of long run time for apigenin owing to its hydrophobicity [Citation28–31]. Therefore, the current method reported in this paper aims to shorten the run time and elute both apigenin and gallic acid either when separately analyzed or when encapsulated in a nanoliposomal formulation. The analytical method was validated, followed by application in the analysis of the entrapment of the co-loaded drugs in the nanoliposomal formulation.
Materials and methods
Materials
Apigenin was purchased from Skin actives scientific company, United States. Gallic acid, orthophosphoric acid, hydrochloric acid, cholesterol, and sodium hydroxide were purchased from Loba Chemical, Jehangir Villa, India. L-α-Phosphatidylcholine soybean lecithin (EPIKURON 200) was kindly gifted by Cargill, Texturizing solutions, Germany. Dimethyldioctadecylammonium bromide (DODAB), Ethanol (HPLC grade > 99.9%), and acetonitrile (HPLC grade > 99.9%) were purchased from Sigma Aldrich Company, USA. Deionized water was obtained from a direct Q-UV 3 millipore device, Millipore SAS, France.
Chromatographic conditions
The chromatographic separation was done on a C18 column (Luna® 3 µm C18 100 Å, LC Column 150 × 4.6 mm, Ea, Phenomenex). Apigenin and gallic acid were chromatographed with a mobile phase consisting of acetonitrile and water containing phosphoric acid 0.2% (67:33, v/v %) using high-performance liquid chromatography (Waters 2690 Alliance HPLC system equipped with a Waters 996 photodiode array detector). The flow rate was 1 ml per minute, the injected volume was 2.5 µL and the chromatographic run time was 2.5 min per sample. The method was monitored at 334 nm for apigenin [Citation32] and 271 nm for gallic acid [Citation33]. All analyses were carried out at 25 °C.
Preparation of standard solutions
An amount of 100 mg of each (apigenin and gallic acid) was transferred to a 100 ml volumetric flask to create a mixture of the two drugs. They were dissolved in ethanol followed by sonication for 10 min, yielding a stock solution of 1 mg/mL of both drugs. Further appropriate dilutions were made to yield the required concentrations (5,10,25,50,100,150,200,250,300,600 μg/mL) for the construction of the calibration curves. The measured areas under the curves were plotted against the corresponding concentrations, and the regression equations were computed.
Validation procedure
The International Conference on Harmonization (ICH) guidelines [Citation34] and the United States Pharmacopeia (USP) requirements were adopted for the validation studies. Various parameters including selectivity, specificity, linearity, accuracy, robustness, precision, and ruggedness (intermediate precision) were evaluated.
To study linearity, the aforementioned dilutions were prepared from a stock drug standard solution. Precision was determined by analyzing a sample six times in two consecutive days (intra-day and inter-day), and calculation of the relative standard deviation (RSD). Ruggedness (intermediate precision) was evaluated by two different analysts, who analyzed the same sample at different day intervals for two days. The accuracy of the method which expresses the closeness of the expected values to the found values was evaluated by calculating the percent recovery of the drugs. The limit of quantification (LOQ) and limit of detection (LOD) were estimated from the standard deviation of residuals (SD) and slope (S) using the following equations: LOD = 3.3(SD/slope) and LOQ = 10 (SD/Slope). The robustness of the method was estimated by testing its tendency to be unaffected by a slight change of the mobile phase concentrations (0.2% phosphoric acid to 0.3% phosphoric acid). The selectivity and specificity were evaluated by comparison of the chromatograms of the test solution and that of a solution exposed to stress conditions by 0.1 N of either HCl or NaOH [Citation35], to test the ability of the method to measure the analyte response in the presence of its potential degradation products.
Preparation of nanoliposomes co-loaded with apigenin and gallic acid
Nanoliposomes were prepared using the ethanol injection method [Citation36,Citation37]. A phospholipid concentration of 20 mg/mL, 0.5 mg/mL cholesterol, 2 mg/mL Dimethyldioctadecylammonium bromide (DODAB), ethanol volume of 1.25 ml containing 0.3 mg of apigenin and 12.5 mg of gallic acid injected by hand press with the antisolvent flow direction, and 5 ml of PBS pH 7.4 were used. The formulations were evaporated using a rotary evaporator (model IKA RV 10 basic, Germany) till complete evaporation of ethanol.
Determination of the entrapment efficiency of apigenin and gallic acid in nanoliposomes
The entrapment efficiency of apigenin and gallic acid in the nanoliposomes was calculated after centrifugation using a Nanosep tube (PALL laboratory Company, USA) [Citation38,Citation39]. The formulations were centrifuged for 30 min at a speed of 15,000 g at room temperature (NEYA 16 R, REMI laboratory instruments, India) [Citation40–42]. The supernatant was collected from the lower compartment, diluted with ethanol and the unentrapped drugs were quantified using the HPLC method described previously. The entrapment efficiency percentage of the drugs was calculated in supernatant according to the following equation:
Results
Chromatograms of apigenin and gallic acid
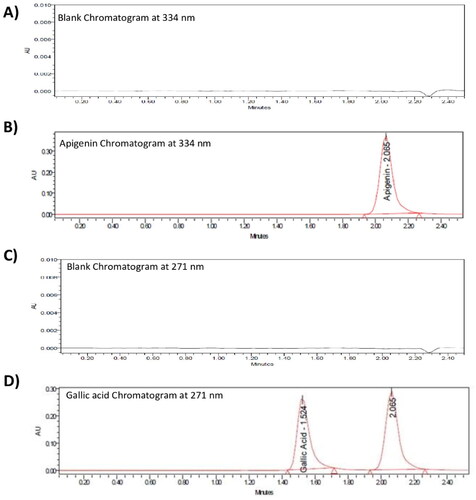
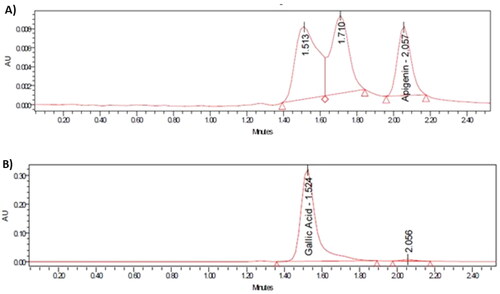
As shown in , both apigenin and gallic acid displayed symmetric peaks using the described assay method, with retention times 2.06 and 1.52 min, respectively. The tailing factors for the apigenin and gallic acid peaks were 1.36 and 1.43 respectively. The relationship between the tested concentrations and peak areas are shown in . As also shown in , there was no interference from formulation components affecting the peak areas of each drug, as the peaks attributed to the lipidic components of liposomes were well-resolved from the apigenin peak.
Validation of the method of assay
Selectivity
The selectivity of the method was confirmed by comparing the chromatograms of the working standard solution before and after the effect of HCl and NaOH addition. As shown in , the retention time, peak resolution, and peak area of apigenin were not affected by treatment with either HCl or NaOH, and likewise for gallic acid in HCl, while showing a significant reduction in the gallic acid area with NaOH only, which was separated without any interference with any peak [Citation43,Citation44], as shown in . This indicates that the proposed HPLC chromatographic method for the determination of apigenin and gallic acid is selective and specific for their simultaneous determination.
Linearity
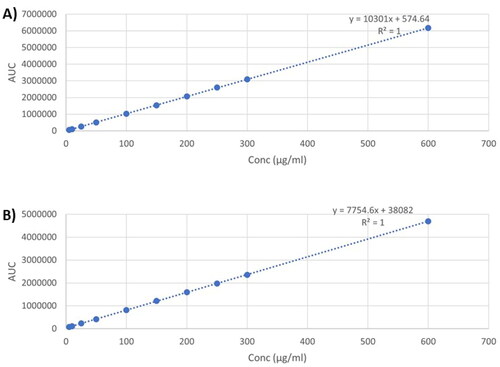
As shown in , the linearity curves for both drugs show a strong linear correlation between the concentration and peak areas in the range of (5-600 µg/mL), with coefficients of determination 1 for both drugs. This suggests the suitability of the method for the quantification of both drugs.
Accuracy
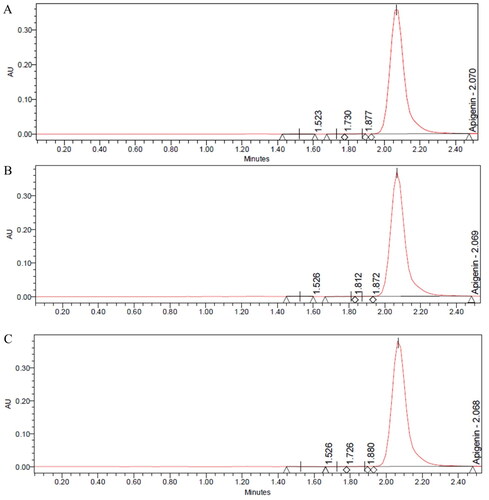
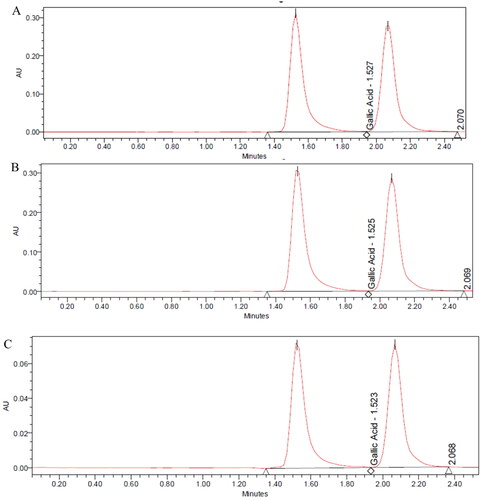
The accuracy results of the standard of apigenin and gallic acid were obtained from 10 samples, each conducted in triplicate. As shown in and , the mean recovery percentages were 99.54 ± 1.16 and 100.48 ± 0.70 for apigenin and gallic acid respectively. The high average recovery for both apigenin and gallic acid suggests that the described HPLC method was accurate and precise for the determination of both drugs.
Table 1. Accuracy data for apigenin (n = 3).
Table 2. Accuracy data for gallic acid (n = 3).
Precision and Ruggedness (intermediate precision)
Results of interday and intraday precision were evaluated by preparing a certain concentration (200 µg/mL) six times for two consecutive days. Results of interday and intraday precision showed a percentage of relative standard deviation (RSD) less than 2% for both drugs as shown in and , hence indicating the precision of the method [Citation45].
Table 3. Precision data for apigenin.
Table 4. Precision data for gallic acid.
The ruggedness of the analytical method was evaluated by analyzing the same concentration tested in the precision experiment but by two different analysts on a different day. Six samples were prepared from the same concentration and were injected as previously described. Results are shown in and .
Table 5. Ruggedness data for apigenin.
Table 6. Ruggedness data for gallic acid.
Determination of the limit of quantification and limit of detection
The limit of detection (LOD) and limit of quantification (LOQ) were calculated to be 0.12 and 4.14 µg/mL respectively for apigenin, and were 0.1 and 3.58 µg/mL respectively for gallic acid, as obtained from the linearity data of both compounds.
Robustness
The effect of variation of mobile phase composition from 0.2% to 0.3% phosphoric acid on apigenin and gallic acid at concentration 200 μg/mL was tested. As shown in , the peak areas of apigenin and gallic acid were not changed after exposure to changes in mobile phase composition.
Table 7. Robustness data for apigenin and gallic acid.
Application of the analytical method in the quantification of entrapment efficiency of apigenin and gallic acid nanoliposomes
The entrapment efficiency of apigenin and gallic acid in the prepared nanoliposomes was 84.74 ± 2.33% and 60.06 ± 3.94% respectively. As shown in , the developed chromatogram showed no interference from liposomal components affecting the peak area of each drug.
Discussion
This work suggests an HPLC method for simultaneous determination of apigenin and gallic acid, with no interference from the liposomal formulation components. The selectivity of the method was confirmed, and the relative standard deviation was less than 2%, indicating the ruggedness of the method [Citation45,Citation46]. The similarity in the peak areas of apigenin and gallic acid after exposure to changes in mobile phase composition suggests the robustness of the method. The tailing factors for the apigenin and gallic acid were within the acceptable range delineated by the ICH guidelines (between 1 and 2) for symmetrical peaks.
Currently, there is an interest in the development of HPLC methods for analysis of drugs within nanoformulations [Citation47]. As obvious from the results, apigenin was entrapped at a higher percent in liposomes owing to its lipophilicity, delineated by its log P value of 3.02 compared with log P value of 0.7 for gallic acid [Citation30]. As a delivery system, liposomes can be considered as one of the most commonly used carriers, owing to their ability to encapsulate both hydrophilic and lipophilic drugs. Owing to the lipophilicity of apigenin, its detection methods suffered from the disadvantage of long run time for apigenin. Therefore, the current method is advantageous in reducing the run time and elute both apigenin and gallic acid either when separately analyzed or when encapsulated in a nanoliposomal formulation, which encourages their co-loading in nanoliposomal formulation while offering means of their simultaneous determination.
Conclusion
As evident from the aforementioned results, the described HPLC method was proven to be convenient, sensitive, and reproducible for the simultaneous identification and quantification of apigenin and gallic acid alone and encapsulated in nanoliposomes. The analytical results were accurate and precise, with good recovery, low limit of detection, and the chromatographic assay was accomplished in less than 3 min.
Acknowledgements
The authors thank Cargill Company for providing free sample of the phospholipids.
Data Availability Statement
Data will be available upon request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ashrafizadeh M, Bakhoda MR, Bahmanpour Z, et al. Apigenin as tumor suppressor in cancers: biotherapeutic activity, nanodelivery, and mechanisms with emphasis on pancreatic cancer. Front Chem. 2020;8:829. doi: 10.3389/fchem.2020.00829.
- Gurung RB, Pandey RP, Sohng JK. Apigenin and Naringenin: Natural Sources, Pharmacology and Role in Cancer Prevention. Chapter: Role of Apigenin in Cancer Prevention. Nova Sci. 2015:107–136.
- Yan X, Qi M, Li P, et al. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:50.
- Imran M, Aslam Gondal T, Atif M, et al. Apigenin as an anticancer agent. Phytother Res. 2020;34(8):1812–1828. doi: 10.1002/ptr.6647.
- Jin X, Yang Q, Zhang Y. Synergistic apoptotic effects of apigenin TPGS liposomes and tyroservatide: implications for effective treatment of lung cancer. Int J Nanomed. 2017;12:5109–5118. doi: 10.2147/IJN.S140096.
- Liu L-Z, Fang J, Zhou Q, et al. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: implication of chemoprevention of lung cancer. Mol Pharmacol. 2005;68(3):635–643. doi: 10.1124/mol.105.011254.
- Pandey R, Gurung R, Sohng J. Apigenin and naringenin: natural sources, pharmacology and role in cancer prevention. Chapter: Dietary Sources, Bioavailability and Biological Activities of Naringenin and Its Derivative. Nova Sci. 2015:107–136.
- Daneshfar A, Ghaziaskar HS, Homayoun N. Solubility of gallic acid in methanol, ethanol, water, and ethyl acetate. J. Chem. Eng. Data. 2008;53(3):776–778. doi: 10.1021/je700633w.
- Cai Y, Zhang J, Chen NG, et al. Recent advances in anticancer activities and drug delivery systems of tannins. Med Res Rev. 2017;37(4):665–701. doi: 10.1002/med.21422.
- Giordani B, Basnet P, Mishchenko E, et al. Utilizing liposomal quercetin and gallic acid in localized treatment of vaginal candida infections. Pharmaceutics. 2020;12(1):9. doi: 10.3390/pharmaceutics12010009.
- Natarajan TD, Ramasamy JR, Palanisamy K. Nutraceutical potentials of synergic foods: a systematic review. J. Ethn. Food. 2019;6(1):1–7. doi: 10.1186/s42779-019-0033-3.
- Santana-Gálvez J, Villela-Castrejón J, Serna-Saldívar SO, et al. Synergistic combinations of curcumin, sulforaphane, and dihydrocaffeic acid against human colon cancer cells. Int J Mol Sci. 2020;21(9):3108. doi: 10.3390/ijms21093108.
- El-Shitany N-A, Abbas AT, Ali SS, et al. Nanoparticles ellagic acid protects against cisplatin-induced hepatotoxicity in rats without inhibiting its cytotoxic activity. Int J Pharmacol. 2019;15(4):465–477. doi: 10.3923/ijp.2019.465.477.
- Song M, Wang J, Lei J, et al. Preparation and evaluation of liposomes Co-loaded with doxorubicin, phospholipase D inhibitor 5-fluoro-2-indolyl deschlorohalopemide (FIPI) and D-Alpha tocopheryl acid succinate (α-TOS) for anti-metastasis. Nanoscale Res Lett. 2019;14(1):138. doi: 10.1186/s11671-019-2964-4.
- Sesarman A, Tefas L, Sylvester B, et al. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine Colon carcinoma microenvironment. Drug Deliv Transl Res. 2019;9(1):260–272. doi: 10.1007/s13346-018-00598-8.
- Akhtar N, Mohammed SA, Singh V, et al. Liposome-based drug delivery of various anticancer agents of synthetic and natural product origin: a patent overview. Pharm Pat Anal. 2020;9(3):87–116. doi: 10.4155/ppa-2019-0020.
- Ochi MM, Amoabediny G, Rezayat SM, et al. In vitro co-delivery evaluation of novel pegylated nano-liposomal herbal drugs of silibinin and glycyrrhizic acid (nano-phytosome) to hepatocellular carcinoma cells. Cell J. 2016;18(2):135–148. doi: 10.22074/cellj.2016.4308.
- You BR, Park WH. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol in Vitro. 2010;24(5):1356–1362. doi: 10.1016/j.tiv.2010.04.009.
- Nasr M, Al-Karaki R. Nanotechnological innovations enhancing the topical therapeutic efficacy of quercetin: a succinct review. Curr Drug Deliv. 2020;17(4):270–278. doi: 10.2174/1567201817666200317123224.
- Nasr M, Wahdan SA. Neuroprotective effects of novel nanosystems simultaneously loaded with vinpocetine and piracetam after intranasal administration. Life Sci. 2019;226:117–129. doi: 10.1016/j.lfs.2019.04.014.
- Amer SS, Nasr M, Mamdouh W, et al. Insights on the use of nanocarriers for acne alleviation. Curr Drug Deliv. 2019;16(1):18–25. doi: 10.2174/1567201815666180913144145.
- Hatem S, Nasr M, Elkheshen SA, et al. Recent advances in antioxidant cosmeceutical topical delivery. Curr Drug Deliv. 2018;15(7):953–964. doi: 10.2174/1567201815666180214143551.
- Bseiso EA, Nasr M, Sammour O, et al. Recent advances in topical formulation carriers of antifungal agents. Indian J Dermatol Venereol Leprol. 2015;81(5):457–463. doi: 10.4103/0378-6323.162328.
- Nasr M, Mansour S, Mortada ND, et al. Vesicular aceclofenac systems: a comparative study between liposomes and niosomes. J Microencapsul. 2008;25(7):499–512. doi: 10.1080/02652040802055411.
- Paini M, Daly SR, Aliakbarian B, et al. An efficient liposome based method for antioxidants encapsulation. Colloids Surf B Biointerfaces. 2015;136:1067–1072. doi: 10.1016/j.colsurfb.2015.10.038.
- Banerjee K, Banerjee S, Das S, et al. Probing the potential of apigenin liposomes in enhancing bacterial membrane perturbation and integrity loss. J Colloid Interface Sci. 2015;453:48–59. doi: 10.1016/j.jcis.2015.04.030.
- Banerjee K, Banerjee S, Mandal M. Enhanced chemotherapeutic efficacy of apigenin liposomes in colorectal cancer based on flavone-membrane interactions. J Colloid Interface Sci. 2017;491:98–110. doi: 10.1016/j.jcis.2016.12.025.
- Arun P, Amol T, Darshan T. Preparation, development and validation of uv spectrophotometric method for the estimation of apigenin in apigenin-hydrogenated soy phosphatidylcholine (HSPC) complex. Int J Pharm Pharm Sci. 2015;7:228–231.
- Kim H-W-MH, Kim H-W-MH, Jung S-HS. Aqueous solubility enhancement of some flavones by complexation with cyclodextrins. Bull Korean Chem Soc. 2008;29:590–594.
- Cheruvu HS, Yadav NK, Valicherla GR, et al. LC-MS/MS method for the simultaneous quantification of luteolin, wedelolactone and apigenin in mice plasma using hansen solubility parameters for liquid-liquid extraction: application to pharmacokinetics of eclipta alba chloroform fraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1081–1082:76–86. doi: 10.1016/j.jchromb.2018.01.035.
- Shetti P, Jalalpure SS. A single robust stability-indicating RP-HPLC analytical tool for apigenin quantification in bulk powder and in nanoliposomes: a novel approach. Futur J Pharm Sci. 2021;7(1):122. doi: 10.1186/s43094-021-00268-6.
- Brito A, Ramirez JE, Areche C, et al. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules. 2014;19(11):17400–17421. doi: 10.3390/molecules191117400.
- Zhang A, Wan L, Wu C, et al. Simultaneous determination of 14 phenolic compounds in grape canes by HPLC-DAD-UV using wavelength switching detection. Molecules. 2013;18(11):14241–14257. doi: 10.3390/molecules181114241.
- Guideline IHT. Validation of analytical procedures: text and methodology. Q2. 2005;50:121–129.
- Nadal JM, Da Graça Toledo M, Pupo YM, et al. A stability-indicating HPLC-DAD method for determination of ferulic acid into microparticles: development, validation, forced degradation, and encapsulation efficiency. J Anal Methods Chem. 2015;2015:286812–286810. doi: 10.1155/2015/286812.
- Gouda A, Sakr OS, Nasr M, et al. Ethanol injection technique for liposomes formulation: an insight into development, influencing factors, challenges and applications. J Drug Deliv Sci Technol. 2021;61:102174. doi: 10.1016/j.jddst.2020.102174.
- Batzri S, Korn ED. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2.
- Cipolla D, Wu H, Gonda I, et al. Aerosol performance and stability of liposomes containing ciprofloxacin nanocrystals. J Aerosol Med Pulm Drug Deliv. 2015;28(6):411–422. doi: 10.1089/jamp.2015.1241.
- Ashraf O, Nasr M, Nebsen M, et al. In vitro stabilization and in vivo improvement of ocular pharmacokinetics of the multi-therapeutic agent baicalin: delineating the most suitable vesicular systems. Int J Pharm. 2018;539(1-2):83–94. doi: 10.1016/j.ijpharm.2018.01.041.
- Joshi M, Patravale V. Formulation and evaluation of nanostructured lipid carrier (NLC)-based gel of valdecoxib. Drug Dev Ind Pharm. 2006;32(8):911–918. doi: 10.1080/03639040600814676.
- Joshi M, Pathak S, Sharma S, et al. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: nanoject. Int J Pharm. 2008;364(1):119–126. doi: 10.1016/j.ijpharm.2008.07.032.
- Vuddanda PR, Rajamanickam VM, Yaspal M, et al. Investigations on agglomeration and haemocompatibility of vitamin E TPGS surface modified berberine chloride nanoparticles. Biomed Res Int. 2014;2014:951942–951911. doi: 10.1155/2014/951942.
- Damle M, Dalavi N. Development and validation of stability indicating HPLC method for determination of ellagic and gallic acid in jambul seed. Int J Appl Sci Biotechnol. 2015;3(3):434–438. doi: 10.3126/ijasbt.v3i3.12908.
- Irakli MN, Samanidou VF, Biliaderis CG, et al. Development and validation of an HPLC-method for determination of free and bound phenolic acids in cereals after solid-phase extraction. Food Chem. 2012;134(3):1624–1632. doi: 10.1016/j.foodchem.2012.03.046.
- Yam MF, Mohamed EAH, Ang LF, et al. A simple isocratic HPLC method for the simultaneous determination of sinensetin, eupatorin, and 3’-hydroxy-5,6,7,4’-tetramethoxyflavone in orthosiphon stamineus extracts. J Acupunct Meridian Stud. 2012;5(4):176–182. doi: 10.1016/j.jams.2012.05.005.
- Kayesh R, Sultan MZ, Rahman A, et al. Development and validation of a RP-HPLC method for the quantification of omeprazole in pharmaceutical dosage form. J. Sci. Res. 2013;5(2):335–342. doi: 10.3329/jsr.v5i2.12779.
- Marques, Shirleen Miriam, Kumar, Lalit, Salwa,. Quality-by-design-based development of an eco-friendly HPLC method for the estimation of nisoldipine in nanoformulations: forced degradation studies and in-vitro release studies. Sustainable Chemistry and Pharmacy 2023;36:101254. doi: 10.1016/j.scp.2023.101254.