Abstract
Background
Though Janus kinase inhibitors such as upadacitinib rapidly relieve itch in atopic dermatitis (AD) patients, how early itch relief impacts later skin clearance is not examined.
Objectives
This study aims to determine if early itch relief by upadacitinib could predict complete skin clearance in later phases.
Methods
This retrospective study involved 105 patients with moderate-to-severe AD treated with upadacitinib 15 mg/day. Eczema area and severity index (EASI), atopic dermatitis control tool, and achievement rate of EASI 100 were evaluated at weeks 4, 12, and 24. The threshold of early peak pruritus-numerical rating scale (PP-NRS) predicting later skin clearance was assessed by area under the receiver-operating characteristic curve, and predictors for EASI 100 achievement were determined by logistic regression analysis.
Results
The rate of achieving EASI 100 at week 24 was extremely higher in patients who achieved week 2 PP-NRS ≤ 1 (42.9%) than in non-achievers (1.4%). The logistic regression analysis showed that the achievement of week 2 PP-NRS ≤ 1 and low body mass index were associated with achievement of EASI 100 at weeks 12 and 24.
Conclusions
The achievement of week 2 PP-NRS ≤ 1 may predict later skin clearance in upadacitinib treatment.
Introduction
Atopic dermatitis (AD) is associated with severe itch (Citation1–3). Itch triggers scratch which disrupts skin barrier and promotes the release of thymic stromal lymphopoietin (TSLP) or interleukin (IL)-33 from epidermis inducing type 2-skewed inflammation, leading to the exacerbation of rash (Citation4).
Conversely, the relief from itch may ameliorate the other symptoms of AD and lead to the clearance of rash, especially the earlier itch relief might expect greater improvement of rash. Janus kinase (JAK) inhibitors, including upadacitinib, abrocitinib, and baricitinib, are significant advancements in the systemic treatment of AD. They have demonstrated rapid itch relief at early points of time (≤ 2 weeks) in both clinical trials (Citation5–9). Post-hoc analysis of phase III JADE COMPARE trial showed that patients who achieved ≥ 4-point improvement of peak pruritus-numerical rating scale (PP-NRS) (PP-NRS4 responders) at week 2 of abrocitinib treatment have higher achievement rates of eczema area and severity index (EASI) 90 and investigator’s global assessment (IGA) 0/1 at week 12 than non-responders (Citation10). The results indicate that early itch relief might predict clear or almost clear skin at later phases of treatment with abrocitinib, and possibly with another JAK1 inhibitor upadacitinib. We previously confirmed that treatment with upadacitinib induced itch relief in precedence of improvement of rash in patients with moderate-to-severe AD in a real-world clinical practice (Citation11–19). A recent study has highlighted the effectiveness of upadacitinib in treating difficult-to-treat areas of AD, such as the eyelids, in patients resistant to dupilumab therapy (Citation20). This evidence further supports the versatility of upadacitinib in managing AD symptoms across various body regions. In this study, we aimed to examine if patients who had rapid itch relief at week 2 of treatment with upadacitinib may attain complete or almost complete clearance of rash at week 12 or 24 in real-world clinical practice.
Methods
Study design and data collection
A retrospective study was conducted at Department of Dermatology in Nippon Medical School Chiba Hokusoh Hospital from August 2021 to April 2023 on 105 patients (aged ≥ 12 years) with moderate-to-severe AD. These patients were diagnosed with AD according to the Japanese Guidelines for Atopic Dermatitis 2021 (Citation21) and were assessed as having moderate-to-severe AD (EASI ≥ 16 or a head-and-neck EASI ≥ 2.4). Patients were included if they had a PP-NRS score ≥ 4-point before treatment. Patients with a baseline PP-NRS score <4 were excluded from the study. All 105 AD patients received oral upadacitinib 15 mg/day in combination with moderate to strongest topical corticosteroids twice daily.
Demographic and clinical data, including sex, age, body mass index (BMI), duration of AD, and history of allergic diseases were recorded for the patients.
This study was conducted in accordance with the Declaration of Helsinki (2004) and was approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital. Written informed consent was provided by the patients.
Measurement of clinical indexes
The patients reported the severity of itch by PP-NRS at weeks 2, 4, 12, and 24 of upadacitinib treatment. The patients also reported their status of disease control using the atopic dermatitis control tool (ADCT) simultaneously. ADCT consists of 6 items of questions regarding symptoms, pruritus, and quality of life (QOL) and patients give a score of 0–4 points for each item with higher scores corresponding to worse disease control (Citation22). The proportion of patients who achieved ≥ 90% or 100% reduction from baseline in EASI (EASI 90 or 100, respectively) was calculated at weeks 4, 12, and 24. Concurrently, the proportion of patients who achieved IGA = 0 (clear) or 1 (almost clear) (IGA 0/1) with ≥ 2-grade reduction of IGA from baseline was also determined.
Statistical analysis
Results are expressed as mean ± standard deviation (SD) for variables with a normal distribution and median and interquartile range for variables with a nonparametric distribution. Differences in measurements at various time points were analyzed using Friedman’s test followed by Bonferroni post hoc test. Differences in frequencies were assessed by Fisher’s exact test. Differences between two groups were analyzed by Mann-Whitney U test. Statistical significance was set at p < 0.05.
The receiver operating characteristic (ROC) analysis was used to assess the predictability of EASI 90, 100, or IGA 0/1 at weeks 12 or 24 by week 2 value or reduction in PP-NRS score. In this analysis, the area under the curve (AUC), sensitivity, and specificity were computed. Predictors for EASI 100 at weeks 4, 12, or 24 were assessed by multiple logistic regression analysis. The analysis included week 2 PP-NRS ≤ 1 achievement as a potential predictor, with results adjusted for age, sex, BMI. Variables with a variance inflation factor >10 were excluded to avoid multicollinearity. In cases of missing data, affected patients were excluded from the analysis to ensure data integrity and accuracy. All statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University).
Results
Demographics and baseline characteristics
Out of the 105 AD patients, 73 patients (69.5%) were male, 31 patients (29.5%) were aged <18 years, and 25 patients (23.8%) had experienced systemic treatment (). The baseline EASI, PP-NRS, or ADCT was median [interquartile range] 23.2 [17.6–32], 8 [7–9], or 14.5 [11–19], respectively.
Table 1. Patient demographics and medical history at baseline (n = 105).
Transition of PP-NRS during upadacitinib treatment
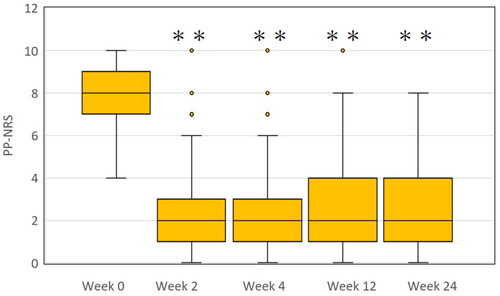
The PP-NRS value was rapidly reduced from median [interquartile range] 8 [7–9] at week 0 to 2 [1–3] at week 2, and plateaued thereafter ().
The optimal threshold of week 2 PP-NRS value to predict skin clearance at weeks 12 or 24
We then analyzed if absolute value of PP-NRS or reduction of PP-NRS from baseline at week 2 may predict the later phases (week 12 or 24) of complete or almost complete clearance of rash (EASI 90, 100, or IGA 0/1) by measuring area under the ROC curve (). The values of AUCs representing the predictability were generally higher using absolute value of PP-NRS (upper three lines) compared to PP-NRS reduction (lower three lines), indicating the superiority of the former. Especially AUC, sensitivity, and specificity in predicting EASI 100 by week 2 PP-NRS values (second line of , ) were higher compared to those in predicting EASI 90 or IGA0/1 (first or third line of , respectively, ), indicating that EASI 100 may be most suitable end-point for prediction by week 2 PP-NRS values. AUC for predicting EASI 100 by week 2 PP-NRS values was 0.849 or 0.861 with cutoff value 0 or 1 of PP-NRS at week 12 or 24, respectively. The threshold of PP-NRS 1-point at week 2, cutoff value for predicting EASI 100 at week 24 is used in further analyses due to the highest AUC. The number of patients who achieved week 2 PP-NRS ≤ 1 (week 2 PP-NRS ≤ 1 achievers) was 35 while that of non-achievers was 70.
Figure 2. Predictability for week 12 eczema area and severity index (EASI) 100 (a), week 12 investigator’s global assessment (IGA) 0/1 (b), week 24 EASI 100 (c) or week 24 IGA 0/1 (d) by week 2 peak pruritus-numerical rating scale (PP-NRS) value in treatment with upadacitinib 15 mg/day as evaluated by area under the receiver operating characteristic curve (AUC).

Table 2. Optimal threshold for week 2 PP-NRS response to predict clearance of rash at weeks 12 or 24.
The transition of rates for achieving PP-NRS ≤ 1 during upadacitinib treatment
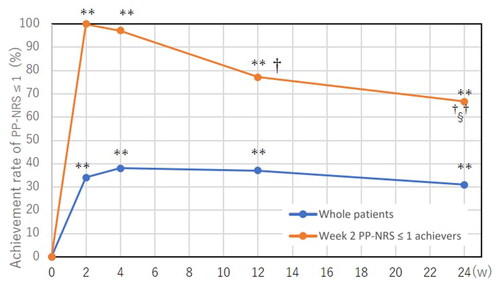
We analyzed the transition of rate for achieving PP-NRS ≤ 1 during upadacitinib treatment (). In whole patients, the rate of achieving PP-NRS ≤ 1 was peaked at week 4 and plateaued thereafter. In week 2 PP-NRS ≤ 1 achievers, the rate of PP-NRS ≤ 1 was slightly reduced at later points of time, weeks 12 or 24.
Figure 3. Transition of achievement rates of peak pruritus-numerical rating scale (PP-NRS) ≤ 1 in whole patients (n = 105) and in achievers of week 2 PP-NRS ≤ 1 (n = 35) during treatment with upadacitinib 15 mg/day. **p < 0.01 versus week 0; †p < 0.05, ††p < 0.01 versus week 2; §p < 0.05 versus week 4, analyzed by Fisher’s exact test.
Comparison of rates for achieving skin clearance between week 2 PP-NRS ≤ 1 achievers and non-achievers
Firstly, the rates of achieving EASI 100, complete skin clearance, were compared between week 2 PP-NRS ≤ 1 achievers (n = 35) and non-achievers (n = 70) (). Week 2 PP-NRS ≤ 1 achievers showed significantly higher rates of EASI 100 compared to non-achievers; the rates of EASI 100 in achievers versus (vs) non-achievers were 14.3% vs 0% (p < 0.05), 28.6% vs 1.4% (p < 0.01), 42.9% vs 1.4% (p < 0.01) at week 4, 12, or 24, respectively. Further, the proportion of week 2 PP-NRS ≤ 1 achievers in patients who accomplished EASI 100 was extremely higher than that in patients who did not; the proportion of week 2 PP-NRS ≤ 1 achievers in EASI 100 accomplishers vs EASI 100 non-accomplishers was 5/5 patients (100%) vs 30/100 patients (30%), 10/11 patients (90.9%) vs 25/94 patients (26.6%), or 15/16 patients (93.8%) vs 20/89 patients (22.5%) at week 4, 12, or 24, respectively. Significant differences in the proportion were observed at all points of time (p < 0.01 for each comparison by Fisher’s exact test). The results support that achievement of week 2 PP-NRS ≤ 1 may predict EASI 100 at later time points.
Figure 4. The comparison of achievement rates for eczema area and severity index (EASI) 100 (a), EASI 90 (b), or investigator’s global assessment (IGA) 0/1 (c) between achievers of week 2 PP-NRS ≤ 1 (n = 35) versus non-achievers (n = 70). *p < 0.05, **p < 0.01 versus non-achievers, analyzed by fisher’s exact test.
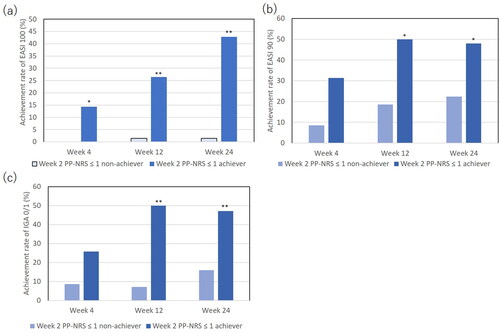
The rates of achieving EASI 90 () or IGA0/1 (), complete or almost complete skin clearance, were also extremely higher in week 2 PP-NRS ≤ 1 achievers than in non-achievers.
The transition of EASI, PP-NRS, and ADCT in week 2 PP-NRS ≤ 1 achievers and non-achievers
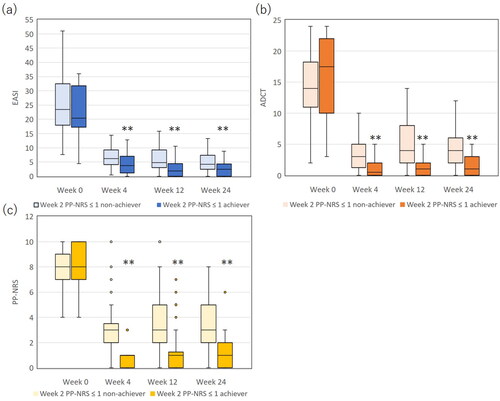
We assessed the transition of EASI, PP-NRS, and ADCT in week 2 PP-NRS ≤ 1 achievers and non-achievers. In both populations, EASI, PP-NRS, and ADCT greatly reduced at week 4 and mostly plateaued thereafter (). Though baseline levels of EASI, PP-NRS, and ADCT were not significantly different between achievers vs non-achievers, values of EASI, PP-NRS, and ADCT were significantly lower in achievers than in non-achievers at weeks 4, 12, and 24 (p < 0.01). The results indicate that while week 2 PP-NRS ≤ 1 achievers maintained the higher improvement of itch and other clinical signs, and better disease control including better QOL at later time points compared to non-achievers, it is important to note that non-achievers also showed a significant clinical response with consistently low EASI scores during treatment.
Figure 5. The comparison of eczema area and severity index (EASI) (a), atopic dermatitis control tool (ADCT) (b) and peak pruritus-numerical rating scale (PP-NRS) (c) between achievers of week 2 PP-NRS ≤ 1 (n = 35) versus non-achievers (n = 70). **, p < 0.01 versus non-achievers, analyzed by Mann-Whitney test.
Predictive factors for achieving EASI 100 at weeks 4, 12, or 24
We finally examine if week 2 PP-NRS ≤ 1 achievement might predict the achievement of EASI 100 at later time points by logistic regression analysis (). The analysis showed that week 2 PP-NRS ≤ 1 achievement and low BMI were associated with achievement of EASI 100 at weeks 12 and 24 but not at week 4; the odds ratio of week 2 PP-NRS ≤ 1 achievement was 38 (95% confidential interval [CI] 3.15–459, p < 0.01) at week 12 and 43.9 (95% CI 4.76–405, p < 0.01) at week 24 while the odds ratio of BMI was 0.503 (95% CI 0.297–0.851, p = 0.0104) at week 12 and 0.782 (95% CI 0.297–0.851, p = 0.0442) at week 24. These results suggest that achievement of week 2 PP-NRS ≤ 1 and low BMI may predict skin clearance at later points of time.
Table 3. The association of each variable with EASI 100 achievement at each time point, analyzed by multiple logistic regression analysis.
Discussion
This study has proven that early itch relief by upadacitinib treatment might predict later skin clearance in patients with AD. Our data confirm that the PP-NRS significantly decreased by week 2, the earliest time point measured, and subsequently plateaued during upadacitinib treatment. The result is in line with those in previous clinical trials (Citation5–7,Citation23) and real-world clinical practice (Citation11, Citation12). The JAK/signal transducer and activator of the transcription (STAT) pathway plays an important role in triggering itch by various cytokines. Upadacitinib blocks the onset of itch by inhibiting JAK1 downstream of receptors for cytokines IL-4, IL-13, IL-31, and TSLP. Similarly to other JAK inhibitors, such as oclacitinib and tofacitinib, upadacitinib might also bind directly to ion channel transient receptor potential vanilloid 1 (TRPV1) and suppress the TRPV1-mediated itch signals independent of JAK1 (Citation24). Additionally, upadacitinib might suppress the IL-31-induced extension of sensory nerves into the epidermis, reducing the sensitivity to pruritogens (Citation25).
The achievement of week 2 PP-NRS ≤ 1 might predict achievement of EASI 100 at weeks 12 and 24, indicating that early itch relief by upadacitinib may be the sign for a potentially high responsiveness to this drug in clearance of rash. In week 2 PP-NRS ≤ 1 achievers, JAK1-dependent cytokines such as IL-4/13/31, or TSLP may account for a large percentage of itch-inducing signals and innervation. The achievers may also highly dependent on those cytokines in the development of other symptoms via abnormal immunity and the skin barrier impairment. The early itch relief may stop the scratching behavior, which may relieve patients from disrupted skin barrier and block the epidermal release of alarmins TSLP or IL-33 triggering type 2 inflammation, intercepting the recurrence of rash, leading to the skin clearance at later time points. Since the achievers of week 2 PP-NRS ≤ 1 may predict complete clearance of rash at later time points, clinicians might recommend such achievers to continue the upadacitinib treatment with assurance, and the achievers might increase the motivation for the treatment. Checking PP-NRS after 2-week trial of upadacitinib can be useful to judge the later treatment outcomes.
The achievers of week 2 PP-NRS ≤ 1 consistently maintained lower EASI, PP-NRS, and ADCT compared to non-achievers, indicating sustained improvement of itch, clinical signs, and QOL. However, this does not diminish the significant clinical response observed in non-achievers, who, despite not achievement of EASI 100, experienced consistent symptom control and improved QOL. This highlights the efficacy of the treatment in a broader patient population.
The logistic regression analysis revealed that BMI was negatively associated with EASI 100 at weeks 12 and 24. Obese patients with high BMI may be associated with the increased volume of drug distribution, which may reduce plasma concentration of upadacitinib compared to patients of normal BMI (Citation26). Further, upadacitinib is slightly more lipophilic (AlogP 2.91) compared to other JAK inhibitors, baricitinib (1.1) or abrocitinib (1.25) (Citation27), and may be more likely to be sequestered by hypertrophic adipose tissues in obese patients, which may also reduce their plasma concentrations of upadacitinib. Thus patients with high BMI may obtain lower plasma concentrations of upadacitinib, and thus less likely to achieve EASI 100. Further, patients with high BMI may have hypertrophic adipose tissues with increased production of inflammatory adipokines, such as tumor necrosis factor-α, IL-6, or leptin counteracting the effectiveness of JAK inhibitors (Citation28). It is also reported that patients with high BMI (≥ 35 kg/m2) was associated with reduced responsiveness to JAK inhibitor tofacitinib in patients with psoriatic arthritis (Citation29), or reduced efficacy of baricitinib in patients with rheumatoid arthritis (Citation30). However, the impact of high BMI on the achievement of EASI75 or EASI90 with upadacitinib was not significant. This indicates that while a high BMI might affect the probability of achievement of EASI 100, it does not preclude a meaningful clinical response in terms of achievement of EASI75 or EASI90.
This study has several limitations. Firstly, the participants of this study were limited to Japanese, and further studies should be performed on the patients with different races. Secondly, we only used 15 mg dose of upadacitinib, and further studies should be performed using 30 mg dose of upadacitinib or the other JAK inhibitors.
Conclusion
In conclusion, achievers of week 2 PP-NRS ≤ 1 by upadacitinib were associated with extremely higher achievement rate of EASI 100 at weeks 12 and 24 compared to non-achievers. Logistic regression analysis showed that achievement of week 2 PP-NRS ≤ 1 and low BMI were associated with EASI 100 at weeks 12 and 24. These findings suggest that early itch relief by upadacitinib may predict later skin clearance in AD patients.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital (protocol code H-2022-945 and February 10, 2022 of approval).
Author contributions
Teppei Hagino conceptualized the study, and mainly organized the manuscript. Mai Yoshida and Risa Hamada performed the statistical analyses. Naoko Kanda supervised the study. Hidehisa Saeki and Eita Fujimoto revised the manuscript.
Supplemental Material
Download Zip (541.6 KB)Disclosure statement
Hidehisa Saeki received a lecture fee and research cost from AbbVie GK. Teppei Hagino and Naoko Kanda received lecture fees from AbbVie GK.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Vilsbøll AW, Anderson P, Piercy J, et al. Extent and impact of inadequate disease control in US adults with a history of moderate to severe atopic dermatitis following introduction of new treatments. Dermatol Ther. 2021;11(2):1–8. doi: 10.1007/s13555-021-00488-x.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006.
- Rerknimitr P, Otsuka A, Nakashima C, et al. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflamm Regen. 2017;37:14.
- Honda T, Kabashima K. Reconciling innate and acquired immunity in atopic dermatitis. J Allergy Clin Immunol. 2020; Apr145(4):1136–1137. doi: 10.1016/j.jaci.2020.02.008.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi: 10.1016/j.jaci.2019.11.025.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/S0140-6736(21)00589-4.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343. doi: 10.1001/jamadermatol.2020.3260.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE Mono-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/S0140-6736(20)30732-7.
- Ständer S, Kwatra SG, Silverberg JI, et al. Early itch response with abrocitinib is associated with later efficacy outcomes in patients with moderate-to-severe atopic dermatitis: subgroup analysis of the randomized phase III JADE COMPARE trial. Am J Clin Dermatol. 2023;24(1):97–107. doi: 10.1007/s40257-022-00738-4.
- Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167. doi: 10.1111/1346-8138.16549.
- Hagino T, Saeki H, Fujimoto E, et al. The eosinophil-to-Lymphocyte ratio acts as an indicator for improvement of clinical signs and itch by upadacitinib treatment in atopic dermatitis. J Clin Med. 2023;12(6):2201. doi: 10.3390/jcm12062201.
- Hagino T, Saeki H, Fujimoto E, et al. The differential effects of upadacitinib treatment on skin rashes of four anatomical sites in patients with atopic dermatitis. J Dermatolog Treat. 2023;34(1):2212095.
- Hagino T, Saeki H, Fujimoto E, et al. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023;50(7):869–879. doi: 10.1111/1346-8138.16763.
- Hagino TY, Hamada R, Fujimoto E, et al. Therapeutic effectiveness of upadacitinib on individual types of rash in japanese patients with moderate-to-severe atopic dermatitis. J Dermatol. 2023;2023:16950. doi: 10.1111/1346-8138.16950.
- Kosaka K, Uchiyama A, Ishikawa M, et al. Real-world effectiveness and safety of upadacitinib in Japanese patients with atopic dermatitis: a two-centre retrospective study. Eur J Dermatol. 2022;32(6):800–802. doi: 10.1684/ejd.2022.4365.
- Chiricozzi A, Ortoncelli M, Schena D, et al. Long-term effectiveness and safety of upadacitinib for atopic dermatitis in a real-world setting: an interim analysis through 48 weeks of observation. Am J Clin Dermatol. 2023;24(6):953–961. doi: 10.1007/s40257-023-00798-0.
- Gargiulo L, Ibba L, Piscazzi F, et al. Effectiveness and safety of upadacitinib for moderate-to-severe atopic dermatitis in a real-world setting: a 52-week retrospective study. J Eur Acad Dermatol Venereol. 2023;2023:19507. doi: 10.1111/jdv.19507.
- Kiefer S, König A, Gerger V, et al. Efficacy and treatment satisfaction of different systemic therapies in children and adolescents with moderate-to-severe atopic dermatitis: a real-world study. J Clin Med. 2023;12(3):820.
- Licata G, Tancredi V, Calabrese G, et al. Atopic dermatitis and difficult-to-treat areas: a case series of four patients with dupilumab-resistant primary atopic blepharitis and successfully treated with upadacitinib. Dermatitis. 2023. doi: 10.1089/derm.2023.0196.
- Saeki H, Ohya Y, Furuta J, et al. Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int. 2022;71(4):448–458. doi: 10.1016/j.alit.2022.06.009.
- Pariser DM, Simpson EL, Gadkari A, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr Med Res Opin. 2020;36(3):367–376. doi: 10.1080/03007995.2019.1699516.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi: 10.1016/S0140-6736(21)00588-2.
- Fukuyama T, Ganchingco JR, Mishra SK, et al. Janus kinase inhibitors display broad anti-itch properties: a possible link through the TRPV1 receptor. J Allergy Clin Immunol. 2017;140(1):306–309.e3. doi: 10.1016/j.jaci.2016.12.960.
- Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol. 2018;27(4):327–331. doi: 10.1111/exd.13533.
- Toussirot E. The interrelations between biological and targeted synthetic agents used in inflammatory joint diseases, and obesity or body composition. Metabolites. 2020; Mar 1310(3):107. doi: 10.3390/metabo10030107.
- Roskoski R.Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2023 update. Pharmacol Res. 2023;187:106552. doi: 10.1016/j.phrs.2022.106552.
- Popko K, Gorska E, Stelmaszczyk-Emmel A, et al. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res. 2010;15(Suppl 2):120–122. doi: 10.1186/2047-783x-15-s2-120.
- Giles JT, Ogdie A, Gomez Reino JJ, et al. Impact of baseline body mass index on the efficacy and safety of tofacitinib in patients with psoriatic arthritis. RMD Open. 2021;7(1):e001486. doi: 10.1136/rmdopen-2020-001486.
- Cristiano AF, Zerbini DM, Arora VK, et al. Effect of BMI on baricitinib efficacy: pooled analysis from two phase 3 rheumatoid arthritis clinical trials. Arthritis Rheumatol. 2016;68:1640.