Introduction
Obesity is one of the main comorbidities associated with psoriasis (Citation1,Citation2). However, recent knowledge on adipose tissue, allow to identify the endocrine role of this organ (Citation2). Adipokines are a functional category of various peptides and proteins involved in cell signaling produced by adipocytes (Citation3), with a role in autoimmune processes (Citation3). It has been showed the immunomodulatory effects of adiponectin (anti-inflammatory role), leptin and resistin (inflammatory role), while other adipokines are currently under investigation (Citation3). Inflammation and immune response are involved in psoriasis pathogenesis, suggesting adipokines as possible actors (Citation4,Citation5). Current literature suggests that adipokines may be crucial in the emergence and advancement of psoriasis and its comorbidities. On consequence, it has been supposed that psoriatic treatments may affect the level of adipokines (Citation3). In this scenario, recent knowledge on the role of interleukin (IL)-23 in psoriasis pathogenesis led to several hypotheses on its possible role on adipokine levels (Citation3). Tildrakizumab is a humanized IgG1/k-type monoclonal antibody that binds the p19 protein subunit of IL23 (Citation6–9). Despite its effectiveness and safety have been widely reported (Citation6–9), its role on adipokine production is unknown. The aim of our study was to evaluate the efficacy of tildrakizumab on psoriatic disease and the adipokine profile, to detect any correlation between the psoriasis improvement and the change in the adipokine profile in overweight/obese patients with moderate-to-severe plaque psoriasis.
Material and methods
A monocentric, prospective, observational study was conducted enrolling patients affected by moderate-to-severe plaque psoriasis. Inclusion criteria were: presence of overweight/obesity (BMI > 25), eligibility for treatment with tildrakizumab. Tildrakizumab was scheduled at the dosage of 100 mg at baseline, week (W)4, and W16. Demographic and clinical features [Psoriasis Area Severity Index (PASI)] were collected for each patient at baseline. PASI and adverse events (AEs) were monitored at W8 and W16. Blood samples were collected at baseline and W16 to assess circulating serum levels of leptin and resistin by using the enzyme-linked immunosorbent assay. Graph Pad Prism 4.0 was used to analyze the collected data, using the paired t-test. A p-value <0.05 was considered statistically significant. The study was conducted in accordance with the Declaration of Helsinki and all patients gave informed consent to participate in the study.
Results
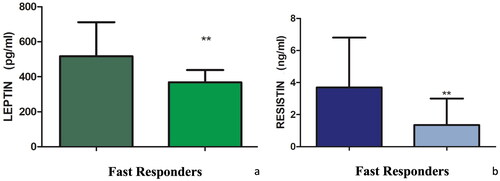
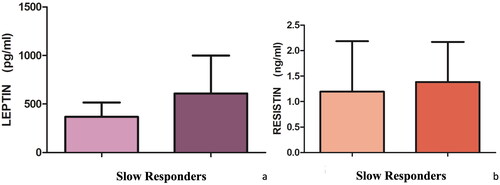
Eleven patients (7 male, 63.6%; mean age 54.5 ± 12.2 years, mean BMI 34.2 ± 7.0 kg/m2) respected the inclusion criteria and completed the follow-up period. A statistically significant reduction in PASI was observed from baseline (16.8 ± 5.7) to W16 (7.6 ± 4.3; p < 0.001). Similarly, a reduction in resistin from baseline (3.6 ± 5.1 ng/mL) to W16 (1.8 ± 2.2 ng/mL) was reported, whereas the value of leptin remains univariate (baseline: 537.5 ± 410.8 pg/mL; W16: 536.3 ± 280.2 pg/mL). However, these changes were not statistically significant. Nevertheless, we noted that there were patients (5, 45.5%) achieving a more rapid response (fast responders) with a reduction in PASI > 50% at W8 and subjects (6, 54.5%) reaching improvement in PASI <40% at W8 (slow responders) compared to the initial period. Correlating the different rapidity and level of clinical response to the adipokines reduction, we noted a significant reduction of the pro-inflammatory adipokines leptin and resistin in fast responder patients (), non-confirmed in slow responder ones (). No AEs were collected.
Discussion
The role of adipokines in psoriasis pathogenesis is debated (Citation1–3). In this context, the potential effect of psoriasis treatments on these cytokines has been supposed. However, data are scant. We performed a prospective study evaluating the efficacy of tildrakizumab on psoriatic disease and the adipokine profile. Despite preliminary, our results showed that the reduction of PASI was associated with the reduction of resistin. However, the association between PASI improvement and leptin reduction was not observed. Nevertheless, we observed that fast responder reached a statistically significant reduction of these adipokines, whereas slow responder patients did not show a significant reduction of leptin and resistin. On consequence, our results showed that targeting IL-23 may lead to the reduction of adipokines, particularly in patients quickly achieving clinical response, suggesting a possible correlation between adipokines levels and therapeutic outcomes. However, the exact mechanism relating adipokines and psoriasis is unknown. Despite limited, our data showed that the use of tildrakizumab may affect the levels of these cytokines. In our opinion, investigating the role of adipokines is crucial to offer patients a personalized approach (Citation10). Main strength is the real-life, prospective design. Main limitation is the low number of patients. Our data showed a possible impact of tildrakizumab on the levels of resistin and leptin, as well as a major reduction in their level in patients achieving a better reduction of the PASI, suggesting the correlation between adipokines reduction and clinical effectiveness. Our preliminary results encourage to investigate the correlation between adipokines and psoriasis, also exploring other cytokines.
Informed consent
The patient gave the consent for publication.
Author contributions
Sara Cacciapuoti: data curation, formal analysis, investigation, visualization, writing-original draft preparation, writing review & editing, supervision. Matteo Megna: data curation, formal analysis, investigation, visualization, writing-original draft preparation, writing—review & editing, supervision. Emanuela Salza: data curation, formal analysis, investigation, visualization, writing-original draft preparation, writing—review & editing, supervision. Luca Potestio: data curation, formal analysis, investigation, visualization, writing-original draft preparation, writing—review & editing, supervision. Giuseppina Caiazzo: data curation, formal analysis, investigation, visualization, writing-original draft preparation, writing—review & editing, supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data that support the findings of this study are available from the corresponding author, upon reasonable request.
Additional information
Funding
References
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1–3. doi: 10.1016/S0140-6736(20)32549-6.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232(6):633–639. doi: 10.1159/000455840.
- Kiełbowski K, Bakinowska E, Ostrowski P, et al. The role of adipokines in the pathogenesis of psoriasis. Int J Mol Sci. 2023;24(7):6390. doi: 10.3390/ijms24076390.
- Filková M, Haluzík M, Gay S, et al. The role of resistin as a regulator of inflammation: implications for various human pathologies. Clin Immunol. 2009;133(2):157–170. doi: 10.1016/j.clim.2009.07.013.
- Brennan AM, Mantzoros CS. Drug insight: the role of leptin in human physiology and pathophysiology–emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2(6):318–327. doi: 10.1038/ncpendmet0196.
- Ruggiero A, Camela E, Potestio L, et al. Drug safety evaluation of tildrakizumab for psoriasis: a review of the current knowledge. Expert Opin Drug Saf. 2022;21(12):1445–1451. doi: 10.1080/14740338.2022.2160447.
- Ruggiero A, Fabbrocicni G, Cacciapuoti S, et al. Tildrakizumab for the treatment of moderate-to-severe psoriasis: results from 52 weeks real-life retrospective study. Clin Cosmet Investig Dermatol. 2023;16:529–536. doi: 10.2147/CCID.S402183.
- Elgaard CDB, Iversen L, Hjuler KF. Guselkumab, tildrakizumab, and risankizumab in a real-world setting: drug survival and effectiveness in the treatment of psoriasis and psoriatic arthritis. J Dermatolog Treat. 2023;34(1):2133531.
- Ruggiero A, Potestio L, Cacciapuoti S, et al. Tildrakizumab for the treatment of moderate to severe psoriasis: results from a single center preliminary real-life study. Dermatol Ther. 2022;35(12):e15941.
- Camela E, Potestio L, Fabbrocini G, et al. The holistic approach to psoriasis patients with comorbidities: the role of investigational drugs. Expert Opin Investig Drugs. 2023;32(6):537–552. doi: 10.1080/13543784.2023.2219387.