Abstract
Dermatofibrosarcoma protuberans (DFSP) is a rare, locally aggressive cutaneous sarcoma with a propensity for recurrence. Its management, particularly in the head and neck (H&N) region, presents unique challenges. This study aimed to evaluate the effectiveness of Mohs micrographic surgery (MMS) compared to wide local excision (WLE) in treating H&N DFSP and its impact on recurrence rates and tissue preservation. A comprehensive search was conducted in PubMed/MEDLINE, yielding 29 relevant studies. We included studies comparing MMS and WLE in adult patients with H&N DFSP and reporting local recurrence outcomes. Data were analyzed using random effects analysis, with a meta-analysis performed for comparative studies. Analysis of studies demonstrated a lower recurrence for MMS. Comparative analysis of five studies involving 117 patients showed a significantly lower recurrence rate in the MMS group (2%) compared to the WLE group (19%). Margin status varied between studies, with some achieving negative margins at shorter distances. In the management of H&N DFSP, MMS has emerged as a superior surgical technique, consistently associated with reduced recurrence rates and the potential for tissue preservation. The adoption of MMS should be considered for its capacity to achieve negative margins with fewer processing steps, particularly in anatomically complex regions like the H&N.
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a low-grade cutaneous sarcoma that can affect the dermis, subcutaneous fat, muscle, and fascia (). It is uncommon, slow-growing, and locally aggressive, with extensive tissue infiltration and a propensity for local recurrence (13–73%) (Citation1–5). With only a few case reports in the literature, DFSP metastasis to cervical lymph nodes remains exceedingly uncommon (Citation6–8). Hematogenous spread with distant metastasis is seen more frequently (10–15%) (Citation9). Therefore, the experience of managing patients with regional extension of DFSP is limited but typically includes radical neck dissection.
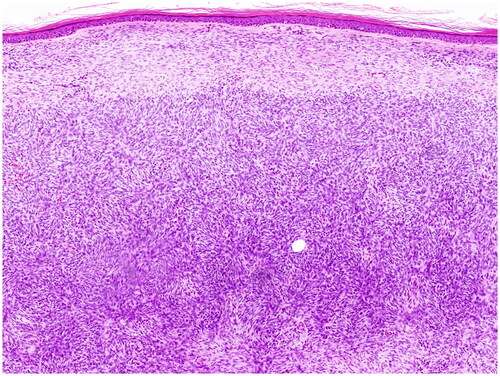
Figure 1. DFSP. Proliferation of uniform spindle cells in the dermis, separated from epidermis by a border zone. (Courtesy: Dr. Daja Šekoranja).
Due to population-based studies conducted in the United States, Canada, and France, the annual incidence of DFSP ranges between 3.0 and 9.3 per one million people, respectively (Citation10,Citation11). Women are 1.14 times more likely to be affected than men. The condition is mostly found on the trunk (42%), followed by the upper and lower extremities (41%), and in a lesser proportion, in the H&N (10–15%), especially on the scalp, supraclavicular region, and forehead (Citation2,Citation3,Citation12,Citation13). Kreicher et al. reported that patients with DFSP who are older, male, black, and have tumor location in the extremities and H&N, have lower survival (Citation10). Go et al. (Citation14) showed a 94% overall survival rate and 99% disease-specific survival rate for the H&N DFSP group at 5 years in 681 SEER patients from 2000 to 2018 (Citation14).
Negative surgical margins have been considered the most important prognostic factor in patients with DFSP, as local recurrence may predispose to distant metastases (Citation15). Consequently, surgical resection with negative margins is the preferred curative treatment (Citation9). Despite wide resection, it is believed that the high recurrence rate is due to the inability of traditional histopathologic methods to evaluate large portions of tissue from the margins, as the tumor creates occult projections in the form of subclinical tentacular extensions into adjacent tissues that may go undetected (Citation15–17).
To achieve a complete resection, it has long been recommended that the first surgical approach use wider margins of 2–3 cm, known in the literature as wide local excision (WLE) (Citation3,Citation16,Citation18). However, as a result of high (13–73%) (Citation1) rate of recurrence rate associated with WLE, Mohs micrographic surgery (MMS) approach is now recommended as the first surgical treatment option, although it is not commonly employed, and normal histological techniques are still used in many regions (Citation19,Citation20). MMS was introduced decades ago in other body sites, which has reduced the rate of DFSP recurrence to <1% (0.0–8.3%) due to intraoperative microscopic control of the surgical margin (Citation3,Citation21–25). Moreover, MMS offers other advantages over WLE for DFSP located in the cervicofacial region, where preservation of healthy tissue without impairing local control is essential (Citation5,Citation15,Citation24–26). In the first case, or classic Mohs, the surgeon acts as or in conjunction with the dermatopathologist to intraoperatively evaluate the frozen pathological sections, whereas in the second case, or Slow-Mohs, the margins are embedded in paraffin and evaluated three-dimensionally without the use of frozen sections. The latter is different from the old technique, which does not examine margins in three dimensions (Citation27). However, some authors consider that it is more difficult to define negative margins with the frozen section technique, which is the most common method for non-melanoma skin cancers, although this is the subject of debate. Better results have been seen with the Slow-Mohs method or paraffin-embedded sections, but it costs more and takes longer (Citation28).
Specific information regarding the outcomes of MMS in H&N DFSP is scant.
This scoping review aims to assess the available evidence regarding the surgical resection technique, specifically MMS in patients with DFSP of the H&N, and its influence on the clinical outcomes of complete resection, tissue preservation, and risk of recurrence. The specific research question is: ‘Is MMS superior to WLE in reducing the rate of local recurrences and preserving adjacent healthy tissue in patients with head and neck DFSP?’
Materials and methods
The search strategy was performed in the PubMed/MEDLINE database using related terms ‘(“Mohs surgery”[MeSH Terms] OR “Mohs”[All Fields]) AND (“head”[All Fields] AND “and”[All Fields] AND “neck”[All Fields]) OR “head and neck”[All Fields] OR (“head”[MeSH Terms] OR “head”[All Fields] OR (“neck”[MeSH Terms] OR “neck”[All Fields]))) AND (“dermatofibrosarcoma”[MeSH Terms] OR (“dermatofibrosarcoma”[MeSH Terms] OR “dermatofibrosarcoma”[All Fields] OR “dermatofibrosarcomas”[All Fields])).’ A ‘snowball’ search was also performed with the references of the identified studies. The last search was made on 1 August 2023. Two authors (PP and AS) performed the search. We searched all primary references for studies comparing MMS and WLE or case series demonstrating the effectiveness of MMS in primary or recurrent tumor cases. We did not contemplate excluding content based on publication year or language.
We examined the full texts of the selected studies and excluded individual case reports. Selection differences were resolved by consensus. We considered studies that included adult patients with DFSP localized to the H&N or information of H&N location in studies on adult patients with DFSP located anywhere in the body), and who underwent surgical resection with MMS and/or compared MMS with WLE and reported local recurrence outcomes. Patients with DFSP in the H&N had to be characterized in detail and with distinction in primary studies. Only studies with fewer than three subjects were excluded. Excel (Microsoft Corp., USA) spreadsheets were used to capture study data.
Random effects analysis was used to calculate the combined incidence (95% confidence interval) for each outcome because this method provides a conservative summary estimate and integrates between-study variance (Citation29). To derive calculations from data containing zero events, a correction of 0.01 was made. The Higgins I2 statistic was used to measure statistical heterogeneity. A forest plot graph was used to display the intervention’s effects. For the analysis of comparative studies, a meta-analysis was conducted with the RevMan V5.4.1 (Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020) software using the risk difference (RD) with random effects method. Due to the study’s design, approval by a research ethics committee was not required.
Results
The initial search resulted in 364 studies. Twenty-nine articles were evaluated after implementing the inclusion and exclusion criteria and reviewing their titles and abstracts ().
There were four systematic reviews, none of which focused explicitly on H&N tumors and none of which included randomized clinical trials (Citation3,Citation17,Citation21,Citation30). Malan et al. (Citation3) included five observational studies with a total of 684 patients, of whom 15.45% were patients with DFSP in the H&N, and is the only meta-analysis describing the specific recurrence rate according to surgical technique, being 38.2% for patients treated with WLE and 16% for those treated with MMS. In 2008 Paradisi et al. (Citation17) compiled data from 29 studies involving 1857 patients, of whom 293 (15.9%) had DFSP of the H&N, with a recurrence rate of 1.9% for patients treated with MMS and 51.8% for WLE patients. None of them were included in the subsequent analyses.
Series of DFSP patients treated with MMS
There were 24 studies having evaluated the recurrence rate in 246 H&N DFSP patients out of a total of 1122 (21.9%) () (Citation22,Citation31–52). Three studies exclusively included 70 patients with H&N malignancies (Citation9,Citation51,Citation52), with the majority (n = 50) requiring between one and four stages for complete resection as primary treatment. Other studies included patients from all locations from which it was possible to extract H&N specific data. No information on the specific sub-sites could be obtained. The studies revealed an overall recurrence frequency of 0% (95% CI: 0–0.4%) ().
Figure 3. Rate of recurrence of DFSP in head and neck area after Mohs micrographic surgery. Case series.
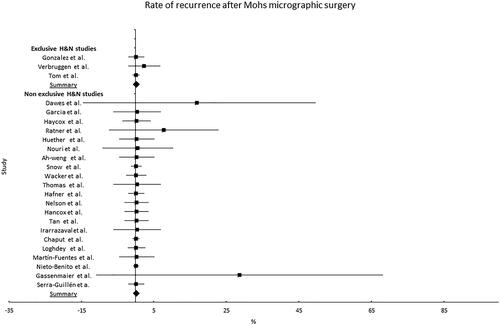
Table 1. Non-comparative studies of the treatment of DFSP of the head and neck with Mohs micrographic surgery (MMS).
Comparative analysis of MMS and WLE recurrence
Five studies comparing MMS to WLE involving 117 patients with H&N cancer were identified (Citation16,Citation17,Citation26,Citation53,Citation54) (). No information on the specific sub-sites could be obtained. In these five studies, the duration of follow-up ranged from 4 to 8.7 years. The mean rate of recurrence was 19% in the WLE group and 2% in the MMS group. The MMS group had a lower recurrence rate (RD −16%) (95% CI −28 to −2%, p = 0.19, I2 = 35%) ().
Figure 4. Comparison of rate of recurrence of DFSP in head and neck area after Mohs micrographic surgery. MMS: Mohs micrographic surgery; WLE: wide local excision.
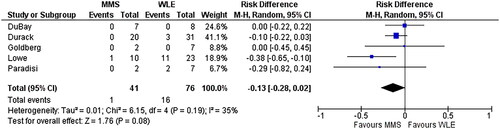
Table 2. Comparative studies between wide local excision (WLE) and Mohs micrographic surgery (MMS) for the treatment of DFSP of the head and neck.
Margin status
Regarding margins, Tom et al. (Citation51) and González et al. (Citation9) reported negative margins in all MMS patients with a tumor distance >2.5 cm, whereas Verbruggen et al. (Citation52) reported a minimal distance of 10 mm. All of these investigations utilized frozen section techniques, and the conclusive results were identical to those reported in definitive paraffin sections.
Discussion
DFSP is a malignant tumor of connective tissue with a favorable prognosis (Citation51). Its traditional treatment is surgical and for many years extensive three-dimensional local resections (distance to the tumor >2 cm) were recommended with the aim of obtaining negative histopathological margins (wide local excision). No consideration to lymph node resection is considered because of the rarity of regional dissemination. Its incidence in the H&N is low (15–20% of all DFSP) (Citation3), but a higher risk of recurrence has been demonstrated than in other body sites, and this has been attributed to the difficulty in achieving negative margins due to anatomical and functional constraints of the surgical site (Citation51). Nonetheless, some authors have proposed a particular histologic behavior using MMS. Gassenmaier et al. (Citation35) performed a retrospective clinicopathologic analysis of 48 patients with DFSP treated by MMS and demonstrated that only 14% of tumors in the H&N region were completely located above the fascia compared to 93% of tumors in the trunk and extremities. This may be due to the thinner skin and subcutaneous cellular tissue in this body region.
Other surgical techniques have been described as alternatives to WLE, including frozen section Mohs micrographic surgery (MMS), modified/Slow-Mohs, complete circumferential deep and peripheral margin assessment (CCPDMA) (Citation41). These aim to assure a negative microscopic margin and, secondarily, to preserve tissue to enhance the esthetic and functional outcomes of resection and facilitate reconstruction. The most recent guidelines have recommended MMS as the treatment of choice (Citation19,Citation20).
The differences in recurrence for patients taken to MMS compared to WLE with DFSP of any location evidenced in the meta-analyses of Malan et al. (Citation3) (2.72 vs. 9.1%), Martin et al. (Citation30) (1.7 vs. 3.73%), and Foroozan et al. (Citation21) (1.1 vs. 6.3%) are clear. The frozen section method was used in most of the studies that were included. However, in some centers, the Slow-Mohs paraffin-embedded definitive section method is more used because frozen Mohs is difficult to process in thick sections (such as the scalp and nape of the neck), restricting the evaluation of the cellular morphology and produces more false negative results (Citation9,Citation16,Citation27,Citation51,Citation52,Citation55). Lee et al. found that frozen MMS is equally effective as paraffin MMS for treating DFSP, however, the paraffin MMS group had a far larger number of patients having previous excision. The paraffin group had longer MMS than the frozen group (Citation28). However, its adoption has been restricted by the complexity of its processing, the need for close to 15–20 h, the excessive cost, and the discomfort of the patient (Citation27,Citation56,Citation57). Gonzalez et al. (Citation9) showed that it is possible to use local anesthesia with excellent results in 70% of cases involving large tumors, despite the fact that some authors have stated that this is unfeasible. Using NCDB data from 2004 to 2016, Desai et al. examined MMS adoption predictors. Academic centers used MMS more than community or integrated network programs when compared to all other treatments. Additionally, Caucasian patients with higher wealth had more MMS (Citation58). Lastly, its application will depend on the availability of the necessary equipment and the institution’s level of expertise. The important question is why MMS isn’t used more often considering its superiority over WLE? Some of the reasons could be that frozen sections may not always be available, the surgical procedure takes longer than expected, MMS can’t be used when the bone is involved and composite removal is needed, or the institution doesn’t have the required experience.
However, information regarding the efficacy of MMS in the treatment of DFSP of the H&N is limited. In evaluating descriptive studies, pertinent evidence supports the routine use of MMS in cases of H&N DFSP. Even though 30% of patients treated corresponded to incomplete resections or recurrences, the frequency of recurrence at more than 5 years is close to 0%. Reviewing the data provided by the few comparative studies reveals a 16% advantage for MMS in terms of recurrence.
Regarding margin size, the results are even more limited. Verbruggen et al. (Citation52) report a mean margin of 1.5 ± 0.57 cm for MMS-managed DFSP patients, while Serra-Guillén et al. (Citation59) calculated a minimum margin of 1.58 cm for complete resection in 222 patients, slightly larger than the overall group’s minimum margin of 1.23 cm. These findings suggest that, with MMS, it is possible to preserve a greater quantity of surrounding tissue, as the standard 3 cm margin recommended for WLE can be reduced (Citation60).
Most scientific literature comes from retrospective studies, which are prone to selection bias. Due to the disease’s rarity, randomized clinical studies comparing H&N DFSP resection regimens are improbable. The studies’ heterogeneity, which includes individuals with original tumors and recurrences of various sizes and sites, makes this more difficult. This study is the most recently published review of scope aimed at obtaining specific information about DFSP in the H&N.
In conclusion, for the H&N presentation of DFSP, the implementation of MMS has reduced the rate of recurrences to a negligible level compared to that of WLE. In addition, considering the anatomical, functional, and esthetic conditions of the cervicofacial region, MMS provides an additional advantage by ensuring negative margins with fewer specimen processing steps and by preserving and optimizing tissue through smaller margins than those described for WLE.
Author contributions
Alvaro Sanabria and Pilar Pinillos: conceptualization, methodology, validation, investigation, data curation, formal analysis, writing–original draft, and writing–review and editing. Carlos Chiesa-Estomba, Orlando Guntinas-Lichius, Luiz P. Kowalski, Antti A. Mäkitie, Karthik N. Rao, and Alfio Ferlito: conceptualization, validation, investigation, data curation, writing–original draft, and writing–review and editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Stojadinovic A, Karpoff HM, Antonescu CR, et al. Dermatofibrosarcoma protuberans of the head and neck. Ann Surg Oncol. 2000;7(9):1–8. doi: 10.1007/s10434-000-0696-3.
- Vitiello GA, Lee AY, Berman RS. Dermatofibrosarcoma protuberans: what is this? Surg Clin North Am. 2022;102(4):657–665. doi: 10.1016/j.suc.2022.05.004.
- Malan M, Xuejingzi W, Quan SJ. The efficacy of Mohs micrographic surgery over the traditional wide local excision surgery in the cure of dermatofibrosarcoma protuberans. Pan Afr Med J. 2019;33:297. doi: 10.11604/pamj.2019.33.297.17692.
- Kim CM, Park TJ, Kim BY, et al. Recurrent dermatofibrosarcoma protuberans of scalp in a distant location 10 years after primary excision. Ann Dermatol. 2018;30(2):226–228. doi: 10.5021/ad.2018.30.2.226.
- Baig IT, Lauck K, Nguyen QD. Retrospective analysis of a modern cohort of dermatofibrosarcoma protuberans from 2000 to 2018. J Cutan Med Surg. 2023;27(2):108–116. doi: 10.1177/12034754221149662.
- Dai Z, He Y, Zhang X, et al. Head-and-neck dermatofibrosarcoma protuberans: survival analysis and clinically relevant immunohistochemical indicators. Oral Dis. 2023. doi: 10.1111/odi.14495.
- Al-Dawsari NA, Al Sheikh SS. Prevalence of dermatofibrosarcoma protuberans in Saudi Arabia over 24 years.: a retrospective single-institution study. Saudi Med J. 2021;42(12):1362–1365. doi: 10.15537/smj.2021.42.12.20210440.
- Lal P, Goel A, Mandal AK. Dermatofibrosarcoma protuberans of scalp with cervical lymph node metastasis. Sarcoma. 2004;8(1):43–45. doi: 10.1080/13577140410001679257.
- González A, Etchichury D, Rivero JM, et al. Treatment of dermatofibrosarcoma of the head and neck with Mohs surgery with paraffin sections. J Plast Reconstr Aesthet Surg. 2021;74(5):1061–1070. doi: 10.1016/j.bjps.2020.10.062.
- Kreicher KL, Kurlander DE, Gittleman HR, et al. Incidence and survival of primary dermatofibrosarcoma protuberans in the United States. Dermatol Surg. 2016;42 Suppl 1:s24–s31. doi: 10.1097/DSS.0000000000000300.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166.
- Mani S, Kumar R, Kakkar A, et al. Recurrent dermatofibrosarcoma protuberans of the head and neck: a case series. Indian J Surg Oncol. 2023;14(1):128–136. doi: 10.1007/s13193-022-01636-1.
- Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol. 2007;56(6):968–973. doi: 10.1016/j.jaad.2006.09.006.
- Go CC, Lahaie Luna GM, Briceño CA. Epidemiological trends and survival outcomes for dermatofibrosarcoma protuberans of the head and neck region. Int J Dermatol. 2023;62(5):664–671. doi: 10.1111/ijd.16459.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9(6):1752. doi: 10.3390/jcm9061752.
- DuBay D, Cimmino V, Lowe L, et al. Low recurrence rate after surgery for dermatofibrosarcoma protuberans: a multidisciplinary approach from a single institution. Cancer. 2004;100(5):1008–1016. doi: 10.1002/cncr.20051.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34(8):728–736. doi: 10.1016/j.ctrv.2008.06.002.
- Chang CK, Jacobs IA, Salti GI. Outcomes of surgery for dermatofibrosarcoma protuberans. Eur J Surg Oncol. 2004;30(3):341–345. doi: 10.1016/j.ejso.2003.12.005.
- National Comprehensive Cancer Network. NCCN guidelines. Dermatofibrosarcoma protuberans; 2024 [cited 2023 Jan 12]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1430
- Saiag P, Grob JJ, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51(17):2604–2608. doi: 10.1016/j.ejca.2015.06.108.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148(9):1055–1063. doi: 10.1001/archdermatol.2012.1440.
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. Results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37(4):600–613. doi: 10.1016/s0190-9622(97)70179-8.
- Gloster HMJr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3 Pt 1):355–374; quiz 375–376. doi: 10.1016/s0190-9622(96)90597-6.
- Lemm D, Mügge L-O, Mentzel T, et al. Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin Oncol. 2009;135(5):653–665. doi: 10.1007/s00432-009-0550-3.
- Mullen JT. Dermatofibrosarcoma protuberans: wide local excision versus Mohs micrographic surgery. Surg Oncol Clin N Am. 2016;25(4):827–839. doi: 10.1016/j.soc.2016.05.011.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with Long-Term follow-up: the Mayo clinic experience. Dermatol Surg. 2017;43(1):98–106. doi: 10.1097/DSS.0000000000000910.
- Breuninger H. Micrographic surgery of malignant skin tumors: a comparison of the frozen technique with paraffin sectioning. Facial Plast Surg. 1997;13(2):79–82. doi: 10.1055/s-2008-1064469.
- Lee SH, Oh Y, Nam KA, et al. Mohs micrographic surgery for dermatofibrosarcoma protuberans: comparison of frozen and paraffin techniques. J Eur Acad Dermatol Venereol. 2018;32(12):2171–2177. doi: 10.1111/jdv.15201.
- Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5(1):52. doi: 10.1186/1756-0500-5-52.
- Martin ECS, Vyas KS, Batbold S, et al. Dermatofibrosarcoma protuberans recurrence after wide local excision versus Mohs micrographic surgery: a systematic review and meta-analysis. Dermatol Surg. 2022;48(5):479–485. doi: 10.1097/DSS.0000000000003411.
- Ah-Weng A, Marsden JR, Sanders DS, et al. Dermatofibrosarcoma protuberans treated by micrographic surgery. Br J Cancer. 2002;87(12):1386–1389. doi: 10.1038/sj.bjc.6600643.
- Chaput B, Filleron T, Le Guellec S, et al. Dermatofibrosarcoma protuberans: margins reduction using slow-Mohs micrographic surgery. Experience with 35 patients. Ann Chir Plast Esthet. 2014;59(4):219–225. doi: 10.1016/j.anplas.2013.11.001.
- Dawes KW, Hanke CW. Dermatofibrosarcoma protuberans treated with Mohs micrographic surgery: cure rates and surgical margins. Dermatol Surg. 1996;22(6):530–534. doi: 10.1111/j.1524-4725.1996.tb00369.x.
- Garcia C, Clark RE, Buchanan M. Dermatofibrosarcoma protuberans. Int J Dermatol. 1996;35(12):867–871. doi: 10.1111/j.1365-4362.1996.tb05053.x.
- Gassenmaier M, Weber E, Leiter U, et al. Micrographic surgery allows fascia preservation in dermatofibro-sarcoma protuberans. Acta Derm Venereol. 2021;101(9):adv00561. doi: 10.2340/00015555-3915.
- Häfner H-M, Moehrle M, Eder S, et al. 3D-histological evaluation of surgery in dermatofibrosarcoma protuberans and malignant fibrous histiocytoma: differences in growth patterns and outcome. Eur J Surg Oncol. 2008;34(6):680–686. doi: 10.1016/j.ejso.2007.07.004.
- Hancox JG, Kelley B, Greenway HTJr. Treatment of dermatofibroma sarcoma protuberans using modified Mohs micrographic surgery: no recurrences and smaller defects. Dermatol Surg. 2008;34(6):780–784. doi: 10.1111/j.1524-4725.2008.34146.x.
- Haycox CL, Odland PB, Olbricht SM, et al. Dermatofibrosarcoma protuberans (DFSP): growth characteristics based on tumor modeling and a review of cases treated with Mohs micrographic surgery. Ann Plast Surg. 1997;38(3):246–251. doi: 10.1097/00000637-199703000-00010.
- Huether MJ, Zitelli JA, Brodland DG. Mohs micrographic surgery for the treatment of spindle cell tumors of the skin. J Am Acad Dermatol. 2001;44(4):656–659. doi: 10.1067/mjd.2001.112381.
- Irarrazaval I, Redondo P. Three-dimensional histology for dermatofibrosarcoma protuberans: case series and surgical technique. J Am Acad Dermatol. 2012;67(5):991–996. doi: 10.1016/j.jaad.2012.03.034.
- Loghdey MS, Varma S, Rajpara SM, et al. Mohs micrographic surgery for dermatofibrosarcoma protuberans (DFSP): a single-centre series of 76 patients treated by frozen-section Mohs micrographic surgery with a review of the literature. J Plast Reconstr Aesthet Surg. 2014;67(10):1315–1321. doi: 10.1016/j.bjps.2014.05.021.
- Martín-Fuentes A, De Eusebio-Murillo E, Herreros CS, et al. Paraffin-embedded micrographic surgery for the treatment of dermatofibrosarcoma protuberans: analysis of 33 patients. Indian J Dermatol Venereol Leprol. 2018;84(3):298–303. doi: 10.4103/0378-6323.190853.
- Nelson RA, Arlette JP. Mohs micrographic surgery and dermatofibrosarcoma protuberans: a multidisciplinary approach in 44 patients. Ann Plast Surg. 2008;60(6):667–672. doi: 10.1097/SAP.0b013e31813376a5.
- Nieto-Benito LM, Ciudad-Blanco C, Sanmartin-Jimenez O, et al. Mohs micrographic surgery in dermatofibrosarcoma protuberans: rate and risk factors for recurrence in a prospective cohort study from the Spanish Registry of Mohs surgery (REGESMOHS) and review of the literature. Exp Dermatol. 2021;30(5):717–722. doi: 10.1111/exd.14291.
- Nouri K, Lodha R, Jimenez G, et al. Mohs micrographic surgery for dermatofibrosarcoma protuberans: University of Miami and NYU experience. Dermatol Surg. 2002;28(11):1060–1064; discussion 1064. doi: 10.1046/j.1524-4725.2002.02084.x.
- Serra-Guillén C, Llombart B, Nagore E, et al. Determination of margins for tumor clearance in dermatofibrosarcoma protuberans: a single-center study of 222 cases treated with modified Mohs surgery. Dermatol Surg. 2022;48(1):51–56. doi: 10.1097/DSS.0000000000003269.
- Snow SN, Gordon EM, Larson PO, et al. Dermatofibrosarcoma protuberans: a report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. Cancer. 2004;101(1):28–38. doi: 10.1002/cncr.20316.
- Tan WP, Barlow RJ, Robson A, et al. Dermatofibrosarcoma protuberans: 35 patients treated with Mohs micrographic surgery using paraffin sections. Br J Dermatol. 2011;164(2):363–366. doi: 10.1111/j.1365-2133.2010.10095.x.
- Thomas CJ, Wood GC, Marks VJ. Mohs micrographic surgery in the treatment of rare aggressive cutaneous tumors: the Geisinger experience. Dermatol Surg. 2007;33(3):333–339. doi: 10.1111/j.1524-4725.2007.33069.x.
- Wacker J, Khan-Durani B, Hartschuh W. Modified Mohs micrographic surgery in the therapy of dermatofibrosarcoma protuberans: analysis of 22 patients. Ann Surg Oncol. 2004;11(4):438–444. doi: 10.1245/ASO.2004.06.014.
- Tom WD, Hybarger CP, Rasgon BM. Dermatofibrosarcoma protuberans of the head and neck: treatment with Mohs surgery using inverted horizontal paraffin sections. Laryngoscope. 2003;113(8):1289–1293. doi: 10.1097/00005537-200308000-00004.
- Verbruggen C, Ricard AS, Cogrel O, et al. [Dermatofibrosarcoma protuberans: surgical margins using slow-Mohs micrographic surgery. A clinical retrospective study about 20 cases]. Ann Chir Plast Esthet. 2018;63(1):47–53. doi: 10.1016/j.anplas.2017.06.005.
- Durack A, Gran S, Gardiner MD, et al. A 10-year review of surgical management of dermatofibrosarcoma protuberans. Br J Dermatol. 2021;184(4):731–739. doi: 10.1111/bjd.19346.
- Goldberg C, Hoang D, McRae M, et al. A strategy for the successful management of dermatofibrosarcoma protuberans. Ann Plast Surg. 2015;74(1):80–84. doi: 10.1097/SAP.0b013e3182898692.
- Farma JM, Ammori JB, Zager JS, et al. Dermatofibrosarcoma protuberans: how wide should we resect? Ann Surg Oncol. 2010;17(8):2112–2118. doi: 10.1245/s10434-010-1046-8.
- Orchard GE, Shams M. Dermatofibrosarcoma protuberans: dealing with slow Mohs procedures employing formalin-fixed, paraffin wax-embedded tissue in a busy diagnostic laboratory. Br J Biomed Sci. 2012;69(2):56–61. doi: 10.1080/09674845.2012.12002437.
- Massey RA, Tok J, Strippoli BA, et al. A comparison of frozen and paraffin sections in dermatofibrosarcoma protuberans. Dermatol Surg. 1998;24(9):995–998. doi: 10.1111/j.1524-4725.1998.tb04293.x.
- Desai AD, Behbahani S, Soliman Y, et al. Factors associated with Mohs micrographic surgery in dermatofibrosarcoma protuberans of the head and neck: a cohort study. Indian J Dermatol Venereol Leprol. 2023;0:1–3. doi: 10.25259/IJDVL_991_2022.
- Serra-Guillén C, Llombart B, Nagore E, et al. Positive margins in excised dermatofibrosarcoma protuberans: a study of 58 cases treated with slow-Mohs surgery. J Eur Acad Dermatol Venereol. 2014;28(8):1012–1015. doi: 10.1111/jdv.12235.
- Chen Y, Jiang G. Association between surgical excision margins and outcomes in patients with dermatofibrosarcoma protuberans: a meta-analysis. Dermatol Ther. 2021;34(4):e14954.