Abstract
Objective
This study aims to assess the efficacy and safety of combining the 308-nm Excimer lamp with Tacrolimus 0.1% ointment, compared to Tacrolimus 0.1% ointment monotherapy, for treating pediatric vitiligo involving less than 10% of the body surface area.
Methods
Fifty pediatric patients with vitiligo were randomly assigned to two groups. Group A received Tacrolimus 0.1% ointment twice daily and Excimer light at 308-nm twice weekly, while Group B received Tacrolimus 0.1% ointment alone, administered twice daily. Repigmentation percentages were evaluated after 30, 90, and 180 days using the rule of nine.
Results
Group A exhibited a significant improvement in repigmentation, increasing from 10% after one month to 65% after six months. In contrast, Group B observed an increase from 10% to 30% over the same timeframe. The efficacy of the treatment was significantly higher in Group A at both the 3-month and 6-month follow-up points (p-value < .001). Moreover, Group A achieved notably higher repigmentation rates in the face, trunk, and lower limbs.
Conclusion
The combination of Tacrolimus and the 308-nm excimer lamp yielded superior repigmentation results compared to Tacrolimus monotherapy in pediatric vitiligo patients. This combined approach may offer an effective new treatment protocol for pediatric vitiligo.
Keywords:
Clinical trial registration:
Introduction
Vitiligo is an acquired skin condition caused by the absence of active melanocytes, resulting in white macules or patches appearing on the skin and mucous membranes (Citation1–2). This disorder is estimated to affect between 0.5 and 2% of the global population (Citation1–2), with nearly half experiencing its symptoms before the age of 20. This statistic underscores vitiligo’s significant presence within pediatric dermatology (Citation2–4). Pediatric vitiligo is relatively prevalent among girls and frequently exhibits an increased segmental presentation (Citation1). However, the condition’s impacts are not solely physical; it also extends into the psychological realm, often causing severe psychosocial distress among both children and adults (Citation1,Citation3,Citation5).
The pathogenesis of vitiligo is largely hypothesized to be autoimmune, with lymphocytes targeting melanocytes (Citation2). Given these immune abnormalities, treatments utilizing immunomodulatory agents, like Tacrolimus, have garnered attention (Citation6–7). Initially developed for atopic dermatitis, Tacrolimus ointment works by inhibiting the production of proinflammatory cytokines (Citation8–9). Its topical application has shown promise in repigmenting areas affected by vitiligo (Citation10–11).
Phototherapy has become a crucial treatment modality for vitiligo. In contrast to oral corticosteroids and cytotoxic drugs—which raise concerns due to their side effects—phototherapy is a relatively safer alternative, particularly for pediatric patients (Citation4). The two primary forms of phototherapy are psoralen-UVA (PUVA) and narrowband UVB. PUVA requires the application of a photosensitizing agent followed by UVA exposure, necessitating protective eyewear and frequent treatment sessions, making it less suitable for children (Citation12).
On the other hand, narrowband UVB therapy has demonstrated safety and efficacy in treating children with generalized vitiligo. It delivers specific wavelengths beneficial for vitiligo treatment while reducing the potential side effects associated with broad-spectrum UVB. However, the long-term safety profile of narrowband UVB continues to be studied (Citation12). The monochromatic excimer lamp (MEL) and 308-nm excimer laser represent significant advancements in phototherapy, delivering concentrated UVB doses to affected skin while sparing healthy tissue (Citation13–14). When combined with agents like topical steroids or calcineurin inhibitors, these technologies have improved treatment outcomes, especially for refractory segmental vitiligo (Citation15–17).
With fewer side effects and less discomfort for patients, these advanced phototherapies are becoming potential gold standards for pediatric vitiligo treatment (Citation13–14). Nonetheless, there is a paucity of studies examining the combined use of Tacrolimus and the 308-nm excimer lamp in pediatric vitiligo patients with less than 10% body surface area affected. Our study seeks to address this gap by assessing the efficacy and safety of combining the 308-nm excimer lamp with Tacrolimus, comparing its outcomes with those of Tacrolimus monotherapy. This dual approach, if successful, could substantially enhance treatment protocols, providing hope for both pediatric and adult patients alike.
Materials and methods
Participants and settings
Participants included patients who visited the dermatology outpatient clinics at King Abdullah University Hospital in Irbid, Jordan, between October 2022 and September 2023. We recruited 50 children, ranging in age from 5 to 17 years, who exhibited non-segmental either generalized or localized vitiligo that affected less than 10% of their body surface area (BSA). All participants underwent a six-week wash-out period before enrollment. Exclusion criteria included diagnoses of Lupus erythematosus, skin dermatoses with the Kobner phenomenon, photosensitivity, skin cancer, or possession of a pacemaker. Both patients and their parents or legal guardians received information about the study’s purpose, confidentiality assurance, and their right to withdraw at any time.
Study design and procedure
In this randomized clinical trial, we allocated patients into two treatment groups of 25 each. Upon consent and assent providing, randomization was used to assign the patients into the treatment groups. Group A received Tacrolimus 0.1% ointment twice daily and excimer light (308-nm) therapy twice weekly. Excimer light starting doses ranged from 50 mJ/cm2 to 400 mJ/cm2, depending on the body site. Post-treatment erythema guided adjustments of subsequent doses as follows:
An additional 50 mJ/cm2 for the next session if erythema was absent or lasted under 24 hours.
No modification to the next session dose if erythema persisted between 24 and 48 hours.
A deduction of 50 mJ/cm2 from the next session if erythema lasted between 48 and 72 hours.
If erythema exceeded 72 hours, or if patients experienced pain and blisters, we postponed the following session until reactions subsided with topical steroids’ assistance. In this situation, the subsequent dose was reduced by 50–100 mJ/cm2.
Group B applied Tacrolimus 0.1% ointment alone, twice daily. Scheduled follow-up visits occurred on days 30, 90, and 180 post-therapy initiation for efficacy assessment via repigmentation area percentage. Using the rule of nines (each arm 9%, chest 9%, stomach 9%, upper back 9%, lower back 9%, each leg 18%, head and neck 9%, and genitalia 1%), two investigators independently estimated the improvement percentage per body site in all patients from the baseline BSA. The overall improvement in repigmentation percentage was then calculated as the average percentage of improvement across all body sites.
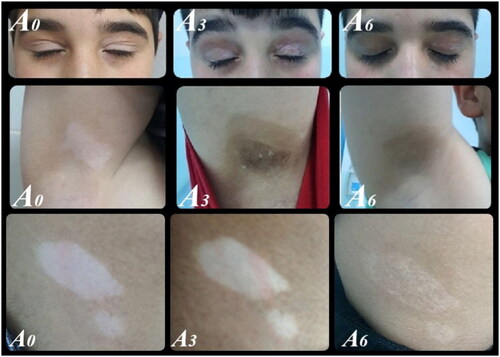
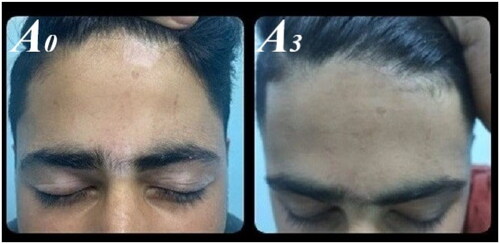
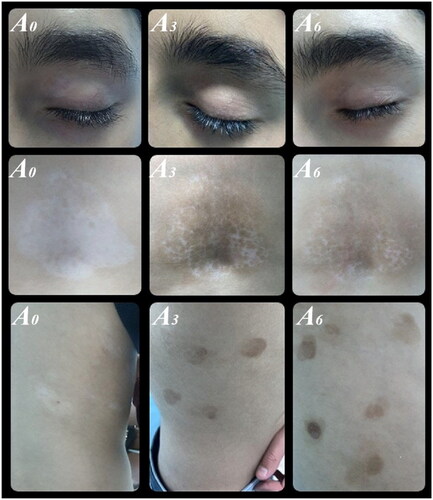
We classified levels of improvement into five categories based on repigmentation percentages: excellent (76%–100%), moderate (51%–75%), mild (26%–50%), minimal (1%–25%), or no response. Serial photographs of vitiligo lesions were taken at baseline and after treatment, documenting any side effects to treatments.
Statistical analysis
For categorical data, including participants’ demographic and clinical characteristics, chi-square tests and percentages were utilized. Medians and interquartile ranges (IQR) were employed for quantitative variables. Analysis was conducted using SPSS version 25.0 (IBM Corp, Armonk, NY, USA), with a significance level set at 5%.
Ethical considerations
The Institutional Review Board (IRB) of Jordan University of Science and Technology approved the study (42/152/2022). All parents or legal guardians provided informed consent for their children’s participation, with confidentiality assured. The study is registered at clinicaltrials.gov under the identifier NCT06035614.
Results
In this randomized clinical trial, both treatment groups A and B consisted of 25 patients each. Group A had 56% female patients, while group B had 60%. The ages of the participants ranged from 5 to 17 years, with a median age of 12 years (IQR= 9–15 yrs) for group A and 14 years (IQR = 10–16 yrs) for group B. No statistically significant differences were observed in terms of gender, age, family history, or side effects across both treatment groups ().
Table 1. Demographics and clinical characteristics of the patients.
In the combined Tacrolimus 0.1% and 308-nm excimer light group (group A), the repigmentation percentage improved from 10% (IQR= 10–27.5%) after 1 month to 65% (IQR= 40–75%) after 6 months ().
In contrast, in the Tacrolimus 0.1% monotherapy group (group B), the repigmentation percentage rose from 10% (IQR= 5–12.5%) after 1 month to 30% (IQR= 21.6–40%) after 6 months. Both treatment groups exhibited significant improvements, with a p-value of <.001. The efficacy (measured by repigmentation percentage) between the groups did not differ significantly after 1 month (p-value = .087). However, group A showed higher efficacy in subsequent follow-ups. By the third month, participants in group A reported a median repigmentation percentage of 40% (IQR = 26.1–55%), while group B reported 15% (IQR = 40–75%). By the sixth month, these values were 65% (IQR = 40–75%) for group A and 30% (IQR = 21.6–40%) for group B. These differences were statistically significant, with a p-value <.001 at both the three- and six-month marks ().
Table 2. Efficacy of each treatment alone and the differences in the efficacy between treatment groups at first, third, and sixth months from baseline.
When categorizing responses by improvement percentages, excellent responses were seen in 4% and 21.7% of group A patients after three and six months, respectively ( and ).
Group B did not report any excellent responses. Moderate responses were observed in 24% and 34.8% of group A patients compared to 8% and 13% in group B at the same time points ().
For patients with skin type III, the combination of Tacrolimus 0.1% and the 308-nm excimer light (group A) showed significantly higher efficacy after three and six months, and for those with skin type IV at all follow-ups. For skin type III, the median improvement percentages after six months were 50% in group A and 27% in group B (p-value = .002). For skin type IV, they were 68% for group A and 29% for group B (p-value = .003) ().
Table 3. The response to treatments according to skin type.
The affected body sites reported were the face, trunk, upper limb (UL), lower limb (LL), and hands & feet for 27, 22, 10, 21, and 20 patients, respectively. Group B did not achieve any excellent responses throughout the therapy. In contrast, after three months, group A reported excellent responses in 29% and 15% of patients for the face and trunk, respectively. After six months, these figures were 36%, 46%, and 33% for the face, trunk, and lower limbs, respectively ().
Group A showed moderate responses across all body sites after both three and six months, whereas group B only reported these for the face, trunk, and lower limbs after six months (). Repigmentation percentages increased significantly from the first to the sixth month for all body sites in both treatments (all p-values < .05). However, for the upper limb in group B, this increase was not significant (p-value = .102). Further analysis showed group A superiority with significant differences in the face (p-value = .001), trunk (p-value = .049), lower limb (p-value = .027), and hands & feet (p-value = .001) after six months of treatment.
Table 4. Percentages of excellent and moderate responses to treatments among patients as an overall and after categoriztion according to various body sites.
As per our study, the use of tacrolimus 0.1% ointment alone was considered entirely safe, as none of the patients in group (B) experienced any adverse reactions. Conversely, in the tacrolimus 0.1% with excimer light at 308-nm group (group A), side effects were observed in 16% of the patients. Nevertheless, these side effects were in the form of mild erythema.
Discussion
Tacrolimus, a calcineurin inhibitor, has been acknowledged for its pivotal role in treating vitiliginous lesions since its endorsement by Grimes et al. in 2002 for adult patients (Citation18). Subsequent studies have further affirmed its efficacy, notably in pediatric patients with vitiligo (Citation10,Citation19,Citation20).
Our study findings are consistent with the extant literature highlighting the efficacy of Tacrolimus as a monotherapy in treating vitiligo, with response rates oscillating between 57.9% and 83.3% (Citation19,Citation21). A previous study (2004–2006), encompassing 100 patients, lends credence to this: 58% of patients under Tacrolimus treatment witnessed successful repigmentation in facial areas, whereas 39% observed improvements in non-facial regions. Of these, 11% achieved total clearance of facial vitiligo, with an additional 10% doing so in non-facial areas (Citation22).
The advent of the 308-nm excimer laser has been a game-changer in the treatment of vitiligo (Citation23–24). Its amalgamation with Tacrolimus has demonstrated promising results, manifesting synergistic benefits (Citation25–28). Our trial echoes the broader scientific consensus, highlighting the duo of Tacrolimus 0.1% ointment and the 308-nm excimer laser as potent treatment options for pediatric vitiligo.
Our one year trial at King Abdullah University Hospital meticulously evaluated two treatment modalities for pediatric non-segmental vitiligo. Fifty participants (aged 5–17) were equally allocated into Group A (median age 12, IQR= 9–15 yrs, 56% females) and Group B (median age 14, IQR = 10–16 yrs, 60% females). Both cohorts exhibited significant repigmentation improvements; however, Group A, which underwent combination therapy, demonstrated higher efficacy. This observation aligns with prior trials, suggesting that vitiligo responds more favorably to the excimer laser when it is coupled with topical treatments like Tacrolimus ointment (Citation21,Citation29,Citation30).
Group A, treated with both Tacrolimus 0.1% ointment and 308-nm excimer light, showcased significant repigmentation improvements between 10% and 65% within the study’s duration. This finding is in harmony with previous research (Citation21,Citation27,Citation31,Citation32). Conversely, Group B, which received only the Tacrolimus 0.1% treatment, experienced a more modest increase from 10% to 30%, especially in the head and neck areas (Citation19–21).
A retrospective review of 57 children undergoing at least three months of Tacrolimus monotherapy showed successful repigmentation in various body areas. The study revealed partial repigmentation in 89% of head and neck cases and 63% of trunk and extremity cases (Citation20), findings that resonate with our results for Group B, further underscoring the efficacy of Tacrolimus as a monotherapy for pediatric vitiligo patients.
Literature, noting repigmentation rates between 57.9% and 83.3% (Citation19,Citation21), highlights the therapeutic prowess of Tacrolimus. Xu et al. reported an impressive 83.3% repigmentation rate (Citation21), while Choi et al. established that Tacrolimus’s effectiveness is not only comparable to topical steroids but also yields quicker response rates (Citation33).
The trial underlines the superior outcomes of combining Tacrolimus with light therapy. It elicited different responses across various skin types and body sites, with Group A exhibiting excellent responses, particularly in patients with skin types III and IV (Citation27,Citation31,Citation32). Treatment responses were significantly noticeable on the face, trunk, and lower limbs, corroborating previous studies noting treatment success variability based on different body sites (Citation34–37).
It’s imperative to acknowledge the negligible side effects recorded, with 16% of Group A reporting side effects and Group B reporting none. The absence of a significant statistical difference between the groups (p-value = .11) accentuates the safety of Tacrolimus, especially in the pediatric demographic.
Tacrolimus and topical corticosteroids like Clobetasol propionate (CP) 0.05% exhibit comparable efficacy rates in pediatric vitiligo treatment, with variations in response rates and time to observable results. Comparative analyses between Tacrolimus and CP 0.05% not only furnish valuable insights but also beckon further explorative studies (Citation22).
Our study’s repigmentation pattern is consistent with existing literature, as the majority of patients demonstrated diffuse repigmentation. The safety profile of Tacrolimus is robust, with minimal adverse events and insignificant clinical side effects noted in children treated for vitiligo affecting less than 20% of the BSA (Citation22).
In conclusion, Tacrolimus 0.1% ointment—whether used as a monotherapy or in combination with a 308-nm excimer lamp—emerges as a promising and safe treatment for pediatric vitiligo (Citation38). The synergy between the two treatments has proven to be effective, and Tacrolimus may also offer protection against UV-induced DNA damage (Citation38). Long-term follow-up data are essential to fully understand and weigh the associated risks and benefits (Citation38). Nevertheless, understanding and balancing the potential risks and benefits necessitate long-term follow-up data (Citation38).
Our study, albeit illuminating, has inherent limitations requiring acknowledgment. The limited sample size (n = 50) warrants cautious extrapolation of results. While the six-month follow-up period provides preliminary insights, it does not guarantee long-term efficacy or late-onset side effects predictions. Conducted in a single center, the study’s applicability might need validation due to the global diversity in ethnicities, skin types, and genetics. Subsequent research endeavors should contemplate larger, diverse participant cohorts, multicenter trials, extended follow-up durations, and the implementation of standardized, objective assessment methodologies. These would not only validate the initial findings but also contribute substantially to the development of pediatric vitiligo treatment protocols.
Conclusion
The trial findings underscore that the combination of a 308-nm excimer lamp and Tacrolimus significantly enhances the effectiveness of pediatric vitiligo treatment compared to Tacrolimus monotherapy. This combined approach not only accelerates the repigmentation process but also yields a higher overall percentage of repigmentation over a six-month period. The efficacy of this combination is particularly pronounced in patients with skin types III and IV. With minimal side effects observed, the combination therapy presents a promising and safe treatment alternative for children suffering from vitiligo. Future research should involve larger cohorts and extended follow-up periods to validate the effectiveness and safety of the combined therapy across diverse pediatric populations. This would aid in the development of improved, patient-centric vitiligo treatment protocols.
Authors contributions
Conceptualization, D.S., F.Q., J.M., Methodology, D.S., S.B., A.F., Formal Analysis S.B., A.F., Data collection D.S., F.Q., J.M., O.T., D.Sa., Resources D.S., F.Q., J.M., A.T., D.Sa., A.A., M.C., Writing original manuscript D.S., F.Q., J.M., S.B., A.F., O.T., D.Sa., A.A., M.C., Review & Editing D.S., F.Q., J.M., S.B., A.F., O.T., D.Sa., A. A., M.C. Supervision of study conduct D.S., F.Q., Project administration and funding D.S., F.Q., A.A.
Supplemental Material
Download PDF (15.7 KB)Acknowledgment
The study was approved and supported by the Deanship of Research at Jordan University of Science & Technology. We would like to thank Clarteis SAS France and The Medical Distributor Ltd., Jordan for their Support in Supplying the Exciplex device (Excimer Device) and the Tacrolimus 0.1% Ointment.
Disclosure statement
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Data availability statement
Data used in the article are available on request from the corresponding author.
Additional information
Funding
References
- Halder RM. Childhood vitiligo. Clin Dermatol. 1997;15(6):1–7. doi: 10.1016/s0738-081x(97)00131-4.
- Kovacs S. Vitiligo. J Am Acad Dermatol. 1998;38(5 Pt 1):647–668. doi: 10.1016/s0190-9622(98)70194-x.
- Mosher DB, Fitzpatrick TB, Ortonne JP, et al. Hypomelanosis and hypermelanosis. In: Fitzpatrick, TB, Eisen, AZ, Wolff, K, Freedberg, IM, Austen, KF, eds. Dermatology in general medicine. New York: McGraw-Hill, 1999: 945–1017.
- Cho S, Kang H-C, Hahm J-H. Characteristics of vitiligo in korean children. Pediatr Dermatol. 2000;17(3):189–193. doi: 10.1046/j.1525-1470.2000.01749.x.
- Lacour J-P. Vitiligo in children: a serious psychological repercussion, in spite of its harmlessness. Rev Prat Med Gen. 1994;8:37–44.
- Grimes PE. Diseases of hypopigmentation. In: Sams WM, Lynch PJ, editors. Principles and practice of dermatology. 2nd ed. New York: Churchill-Livingstone; 1996. p. 873–885.
- Grimes PE. Vitiligo: an overview of therapeutic approaches. Dermatol Clin. 1993;11(2):325–338. doi: 10.1016/S0733-8635(18)30271-7.
- Reitamo S, Wollenberg A, Schöpf E, et al. Safety and efficacy of one year of tacrolimus ointment monotherapy in adults with atopic dermatitis. Arch Dermatol. 2000;136(8):999–1006. doi: 10.1001/archderm.136.8.999.
- Lawrence I. Tacrolimus (FK 506) experience in dermatology. Dermatol Ther. 1998;5:74–84.
- Lepe V, Moncada B, Castanedo-Cazares JP, et al. A double blind randomized trial of 0.1% tacrolimus vs 0.05% clobetasol for the treatment of childhood vitiligo. Arch Dermatol. 2003;139(5):581–585. doi: 10.1001/archderm.139.5.581.
- Alshiyab D, Al-Qarqaz F, Ba-Shammakh S, et al. Comparison of the efficacy of tacrolimus 0.1% ointment vs calcipotriol/betamethasone in combination with NBUVB in treatment of vitiligo. J Dermatolog Treat. 2023;34(1):2252119. doi: 10.1080/09546634.2023.225211.
- Njoo MD, Spuls PI, Bos JD, et al. Nonsurgical repigmentation therapies in vitiligo. Meta-analysis of the literature. Arch Dermatol. 1998;134(12):1532–1540. doi: 10.1001/archderm.134.12.1532.
- Le Duff F, Fontas E, Giacchero D, et al. 308-nm excimer lamp vs. 308-nm excimer laser for treating vitiligo: a randomized study. Br J Dermatol. 2010;163(1):188–192. doi: 10.1111/j.1365-2133.2010.09778.x.
- Do JE, Shin JY, Kim DY, et al. The effect of 308nm excimer laser on segmental vitiligo: a retrospective study of 80patients with segmental vitiligo. Photodermatol Photoimmunol Photomed. 2011;27(3):147–151. doi: 10.1111/j.1600-0781.2011.00587.x.
- Bae JM, Yoo HJ, Kim H, et al. Combination therapy with 308-nm excimer laser, topical tacrolimus, and short-term systemic corticosteroids for segmental vitiligo: a retrospective study of 159 patients. J Am Acad Dermatol. 2015;73(1):76–82. doi: 10.1016/j.jaad.2015.04.008.
- Passeron T, Ortonne JP. Use of the 308-nm excimer laser for psoriasis and vitiligo. Clin Dermatol. 2006;24(1):33–42. doi: 10.1016/j.clindermatol.2005.10.024.
- Li L, Liang Y, Hong J, et al. The effectiveness of topical therapy combined with 308-nm excimer laser on vitiligo compared to excimer laser monotherapy in pediatric patients. Pediatr Dermatol. 2019;36(1):e53–e55.
- Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol. 2002;47(5):789–791. doi: 10.1067/mjd.2002.126250.
- Kanwar AJ, Dogra S, Parsad D. Topical tacrolimus for treatment of childhood vitiligo in Asians. Clin Exp Dermatol. 2004;29(6):589–592. doi: 10.1111/j.1365-2230.2004.01632.x.
- Silverberg NB, Lin P, Travis L, et al. Tacrolimus ointment promotes repigmentation of vitiligo in children: a review of 57 cases. J Am Acad Dermatol. 2004;51(5):760–766. doi: 10.1016/j.jaad.2004.05.036.
- Xu AE, Zhang DM, Wei XD, et al. Efficacy and safety of tacrolimus cream 0.1% in the treatment of vitiligo. Int J Dermatol. 2009;48(1):86–90. Jan). doi: 10.1111/j.1365-4632.2009.03852.x.
- Ho N, Pope E, Weinstein M, et al. A double-blind, randomized, placebo-controlled trial of topical tacrolimus 0·1% vs. clobetasol propionate 0·05% in childhood vitiligo. Br J Dermatol. 2011;165(3):626–632. doi: 10.1111/j.1365-2133.2011.10351.x.
- Park KK, Liao W, Murase JE. A review of monochromatic excimer light in vitiligo. Br J Dermatol. 2012;167(3):468–478. doi: 10.1111/j.1365-2133.2012.11008.x.
- Hong SB, Park HH, Lee MH. Short-term effects of 308-nm xenon-chloride excimer laser and narrow-band ultraviolet B in the treatment of vitiligo: a comparative study. J Korean Med Sci. 2005;20(2):273–278. doi: 10.3346/jkms.2005.20.2.273.
- Lan CC, Chen GS, Chiou MH, et al. FK506 promotes melanocyte and melanoblast growth and creates a favorable milieu for cell migration via keratinocytes: possible mechanisms of how tacrolimus ointment induces repigmentation in patients with vitiligo. Br J Dermatol. 2005;153(3):498–505. doi: 10.1111/j.1365-2133.2005.06739.x.
- Lee KY, Jeon SY, Hong JW, et al. Endothelin-1 enhances the proliferation of normal human melanocytes in a paradoxical manner from the TNF-alpha-inhibited condition, but tacrolimus promotes exclusively the cellular migration without proliferation: a proposed action mechanism for combination therapy of phototherapy and topical tacrolimus in vitiligo treatment. J Eur Acad Dermatol Venereol. 2013;27(5):609–616. doi: 10.1111/j.1468-3083.2012.04498.x.
- Passeron T, Ostovari N, Zakaria W, et al. Topical tacrolimus and the 308-nm excimer laser: a synergistic combination for the treatment of vitiligo. Arch Dermatol. 2004;140(9):1065–1069. doi: 10.1001/archderm.140.9.1065.
- Kawalek AZ, Spencer JM, Phelps RG. Combined excimer laser and topical tacrolimus for the treatment of vitiligo: a pilot study. Dermatol Surg. 2004;30(2):130–135. doi: 10.1097/00042728-200402000-00002.
- Bae JM, Hong BY, Lee JH, et al. The efficacy of 308-nm excimer laser/light (EL) and topical agent combination therapy versus EL monotherapy for vitiligo: a systematic review and meta-analysis of randomized controlled trials (RCTs). J Am Acad Dermatol. 2016;74(5):907–915. doi: 10.1016/j.jaad.2015.11.044.
- Khullar G, Kanwar AJ, Singh S, et al. Comparison of efficacy and safety profile of topical calcipotriol ointment in combination with NB- UVB vs. NB-UVB alone in the treatment of vitiligo: a 24-week prospective right-left comparative clinical trial. J Eur Acad Dermatol Venereol. 2015;29(5):925–932. doi: 10.1111/jdv.12726.
- Lotti T, Buggiani G, Troiano M, et al. Targeted and combination treatments for vitiligo. Comparative evaluation in 458 subjects. Dermatol Ther. 2008;21(1):S20–S26. doi: 10.1111/j.1529-8019.2008.00198.x.
- Fai D, Cassano N, Vena GA. Narrow-band UVB phototherapy combined with tacrolimus ointment in vitiligo: review of 110 patients. J Eur Acad Dermatol Venereol. 2007;21(7):916–920. doi: 10.1111/j.1468-3083.2006.02101.x.
- Choi CW, Chang SE, Bak H, et al. Topical immunomodulators are effective for treatment of vitiligo. J Dermatol. 2008;35(8):503–507. doi: 10.1111/j.1346-8138.2008.00511.x.
- Baltás E, Nagy P, Bónis B, et al. Repigmentation of localized vitiligo with the xenon chloride laser. Br J Dermatol. 2001;144(6):1266–1267. doi: 10.1046/j.1365-2133.2001.04248.x.
- Taneja A, Trehan M, Taylor CR. 308-nm excimer laser for the treatment of localized vitiligo. Int J Dermatol. 2003;42(8):658–662. doi: 10.1046/j.1365-4362.2003.01997.x.
- Ostovari N, Passeron T, Zakaria W, et al. Treatment of vitiligo by 308-nm excimer laser: an evaluation of variables affecting treatment response. Lasers Surg Med. 2004;35(2):152–156. doi: 10.1002/lsm.20057.
- Hartmann A, Bröcker EB, Hamm H. Occlusive treatment enhances efficacy of tacrolimus 0.1% ointment in adult patients with vitiligo: results of a placebo-controlled 12-months prospective study. Acta Derm Venereol. 2008;88(5):474–479. doi: 10.2340/00015555-0464.
- Tran C, Lübbe J, Sorg O, et al. Topical calcineurin inhibitors decrease the production of UVB-induced thymine dimers from hairless mouse epidermis. Dermatol. 2005;211(4):341–347. doi: 10.1159/000088505.