Abstract
Background
Sebum physiology and its contributions to acne vulgaris (AV) pathophysiology have been long debated. Within the pilosebaceous unit, androgens drive sebocyte production of sebum, comprising mono-, di-, and triglycerides (the latter converted to fatty acids); squalene; cholesterol; cholesterol esters; and wax esters. Upon release to the skin surface, human sebum has important roles in epidermal water retention, antimicrobial defenses, and innate immune responses.
Aims
Alterations in sebum alone and with other pathogenic factors (inflammation, follicular hyperkeratinization, and Cutibacterium acnes [C. acnes] proliferation) contribute to AV pathophysiology. Androgen-driven sebum production, mandatory for AV development, propagates C. acnes proliferation and upregulates inflammatory and comedogenic cascades.
Results
Some sebum lipids have comedogenic effects in isolation, and sebum content alterations (including elevations in specific fatty acids) contribute to AV pathogenesis. Regional differences in facial sebum production, coupled with patient characteristics (including sex and age), help exemplify this link between sebum alterations and AV lesion formation.
Conclusions
To date, only combined oral contraceptives and oral spironolactone (both limited to female patients), oral isotretinoin and topical clascoterone (cortexolone 17α-propionate) modulate sebum production in patients with AV. A better understanding of mechanisms underlying sebaceous gland changes driving AV development is needed to expand the AV treatment armamentarium.
1. Introduction
Acne vulgaris (AV) is a chronic inflammatory and immune-mediated skin condition characterized by the presence of noninflammatory (open and closed comedones) and inflammatory (papules, pustules, and nodules) lesions, primarily on the face, chest, upper back, and shoulders (Citation1,Citation2). AV is the eighth most common disease among all diseases globally and can occur in individuals of any age, race, or sex/gender (Citation1,Citation3,Citation4). The high prevalence of AV, coupled with the physical, social, psychological, and economic burdens experienced by patients, highlights the significant global disease burden (Citation5).
AV is a multifactorial disease with 4 key interrelated processes driving pathophysiology: inflammation, increased sebaceous gland activity and sebum production, follicular hyperkeratinization, and colonization of proinflammatory strains of Cutibacterium acnes (C. acnes) (Citation1,Citation6,Citation7). Androgens are sex hormones that mediate excessive sebaceous gland activity and sebum production, representing the earliest step in AV lesion formation (Citation8–12). A growing body of evidence also supports that alterations in sebum composition are important for AV development (Citation13–16).
Commonly recommended therapies that are consistently mentioned in publications on AV management include retinoids, benzoyl peroxide, antibiotics, and hormonal therapies, all of which target 1 or more of these pathogenic factors (Citation2,Citation6,Citation17). To date, only hormonal therapies and isotretinoin (a retinoid) are shown to address increased sebum production in AV (Citation6). Systemic antiandrogenic therapies can induce feminization in male patients (e.g., gynecomastia), may elicit certain side effects in females, and are not recommended for use in pregnant patients (Citation1,Citation6,Citation18–20). The risk of teratogenicity with isotretinoin contraindicates this treatment for patients who are pregnant and necessitates measures to completely avoid pregnancy in women undergoing oral isotretinoin therapy (Citation21). Therefore, novel treatment options are needed that modulate sebaceous gland activity and have acceptable safety profiles in both males and females (Citation6,Citation22).
This review provides an overview of the role of sebum in the pathophysiology of AV from both compositional and quantitative perspectives, discusses factors that influence these changes in sebum, and touches upon sebum-targeting therapeutic options for patients with AV.
2. Sebaceous immunobiology
The sebaceous gland is an exocrine organ composed primarily of sebocytes with the highest density on the scalp, face, upper chest, and back (Citation8,Citation23–25). Sebaceous glands develop alongside hair follicles in the mid-dermis with an outlet emptying into the follicular canal, which in turn opens to the skin surface (collectively known as the pilosebaceous unit) (Citation24). Although the number of sebaceous glands remains the same over time, the size of these glands increases with age (Citation24,Citation26). Sebaceous gland activity has an initial increase at birth and peaks again during adrenarche and subsequent puberty due to the stimulatory effects of circulating sex hormones, primarily androgens (Citation8,Citation27–29).
Sebaceous glands comprise undifferentiated, differentiating, and mature sebocytes (Citation25). Differentiating sebocytes synthesize and accumulate sebum in cytoplasmic droplets (Citation25,Citation30). Sebocyte proliferation and sebum production are modulated by several cell signaling pathways involving androgens, peroxisome proliferator activator receptor (PPAR) ligands, liver-X receptor ligands, retinoids, and vitamin D (Citation31–35). Mature sebocytes undergo programmed cell death resulting in their disintegration and the release of sebum into the follicular canal, a form of holocrine secretion (Citation36). The lifespan of a sebocyte is 7–14 days in mouse tissue (Citation37) and 21–25 days in human tissue from the onset of cell division to the point of disintegration and holocrine secretion (Citation12,Citation38). Sebum is released onto the skin surface, which is a distinctly different skin compartment than the well-organized intercellular lipid membrane of the stratum corneum, composed predominantly of ceramides, fatty acids, and cholesterol (Citation24,Citation39). Sebum production increases during puberty, in parallel with increased androgen levels, and remains relatively stable from the end of puberty through mid-adulthood, before declining later in life (Citation8).
The main contents of human sebum and their relative sebum weight percentages are triglycerides, diglycerides, and free fatty acids (57.5%); wax esters (26%); squalene (12%); and cholesterols (1.5%) (Citation12,Citation40). The physiologic role of sebum has been a subject of discussion and debate for several decades (Citation12,Citation30,Citation41,Citation42). The functions of sebum, some of which were mentioned above, include lubrication of skin and hair, maintenance of skin hydration, antimicrobial effects, antioxidant delivery (e.g., tocopherol) into upper skin layers, and innate immune responses (pattern receptor recognition, antimicrobial peptide activity) (Citation12,Citation43). Sebum can also penetrate the dermis to modulate the function of keratinocytes and immune cells (Citation8,Citation44,Citation45). Some fatty acids in sebum can stimulate antibacterial responses (Citation46). For example, sebum lipids, including oleic acid and squalene, can increase the phagocytic activity of macrophages toward C. acnes (Citation45).
3. Sebum in AV and other disorders
In the context of AV, sebum alone and in coordination with other pathogenic factors can contribute to AV pathogenesis () (Citation6,Citation7,Citation43). The earliest studies of human sebum, published in the late 1960s/early 1970s, demonstrated that almost all sebum lipids have comedogenic effects (Citation49,Citation50). Sebum monounsaturated fatty acids, squalene, and triglycerides have the highest comedogenicity potential, whereas sebum waxes and sterols are only weakly comedogenic (Citation49). Further, sebum unsaturated fatty acids accelerate keratinocyte differentiation and interfere with follicular keratinization, leading to pore blockage and lesion formation (Citation44,Citation51,Citation52). Indeed, closed comedones (whiteheads) and open comedones (blackheads) are attributed at least partially to the comedogenic effects of fatty acids in sebum (Citation49,Citation52).
Figure 1. Contribution of sebum to acne pathophysiology. Acne pathophysiology is driven by 4 key factors: alteration in sebaceous immunobiology (excessive sebum production and alterations in sebum composition), inflammation, follicular hyperkeratinization, and colonization of Cutibacterium acnes (Citation6,Citation47,Citation48). Solid lines indicate an impact of sebum on the other 3 acne pathogenic factors, whereas dashed lines indicate the inverse.
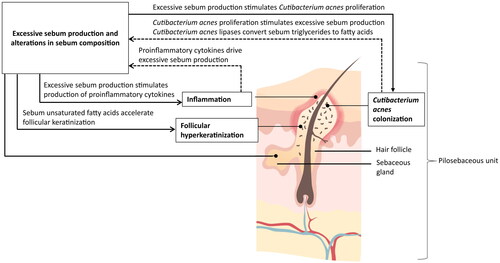
In addition to its role in comedone formation, sebum can promote inflammation through multiple pathways (Citation43). Sebum provides the nutrient source and follicular microenvironment for C. acnes proliferation, and as such, C. acnes is most abundant in areas with a high sebum concentration (Citation53). Increased sebum production and alterations in the sebum lipid profile can therefore stimulate C. acnes growth (Citation22,Citation54,Citation55), which in turn stimulates the production of proinflammatory cytokines by sebocytes and other cells within the pilosebaceous unit and increases sebum production (Citation7,Citation8,Citation43). Finally, sebum also promotes the expression of proinflammatory cytokines, including interleukin (IL)-1, leading to hyperkeratinization and supporting the inflammatory potential of sebum lipids independent of other pathogenic factors (Citation7,Citation51). These studies illustrate both the importance of increased sebum production as an early step in AV formation and the interrelation of the 4 key AV pathogenic factors (Citation6,Citation8).
More recently, a multitude of acquired or congenital inflammatory disorders of the skin (e.g., seborrheic dermatitis, rosacea, and atopic dermatitis) and hair (e.g., scarring alopecia) and endocrine or neuroendocrine disorders (e.g., polycystic ovary syndrome and Parkinson’s disease) have been attributed to sebaceous gland dysfunction (Citation22,Citation56). Therefore, targeting changes in sebaceous gland activity and sebum composition can have widespread therapeutic implications beyond AV treatment (Citation22).
3.1. Sebum quantity
The role of excessive sebum production (seborrhea) in AV pathophysiology has been well established as an important initial step in AV formation, with early observations of increased sebum excretion in patients with AV compared with those without the disease (Citation10,Citation57–59). Further, treatment-mediated AV remission is also associated with reduced sebum excretion rates (Citation60,Citation61). This relationship between seborrhea and AV is also evident based on the predilection for AV to occur in regions of high sebum production (seborrheic zones), including the face (99% of cases), but also the back and chest (90% and 70% of cases, respectively) (Citation23).
Androgens, particularly testosterone and dihydrotestosterone, regulate sebocyte proliferation, lipid synthesis, and differentiation and drive sebum production (Citation8,Citation9,Citation11,Citation62). Androgens are produced by reproductive organs but, importantly, can also be synthesized within the sebaceous gland from adrenal precursors (dehydroepiandrosterone and androstenedione) (Citation8,Citation63–65). Increased circulating androgen levels are observed in males and females during puberty and correlate with the onset of AV, the appearance of enlarged sebaceous glands, and increased sebum production (Citation28,Citation29).
Increased sebum availability, coupled with hyperkeratinization within the microcomedone, provides the lipid-rich anaerobic environment necessary for C. acnes proliferation (Citation54,Citation55). The C. acnes biofilm penetrates sebum to act as an adhesive for corneocytes (terminally differentiated keratinocytes) and facilitates comedone formation (Citation66,Citation67). In turn, C. acnes lipases convert sebum triglycerides into fatty acids, which can stimulate follicular keratinization and inflammatory mediators (Citation44,Citation51,Citation55). C. acnes also activates the corticotropin-releasing hormone and Christie-Atkins-Munch-Peterson factor pathways to stimulate sebum production and amplification of an inflammatory response, including the production of IL-6 and IL-8 within the sebaceous gland (Citation67–69).
Despite the indispensable role of seborrhea in AV pathophysiology, this alone is not sufficient to trigger AV, as demonstrated by the successful treatment of AV with therapies such as antibiotics, which do not target excessive sebum production (Citation6,Citation17). Several studies have identified the differences in sebum lipid composition in patients with AV (Citation14,Citation15,Citation70,Citation71).
3.2. Sebum quality
In patients with AV, alterations in the type and arrangement of fatty acids have been observed (Citation14,Citation15,Citation71). Levels of linoleic acid, an essential fatty acid that is the precursor to squalene and wax esters, are lower in AV sebum (Citation70,Citation71). Depletion of linoleic acid contributes to follicular hyperkeratosis, a key step in comedone formation, as well as impairment in epidermal barrier function (Citation72,Citation73), which could increase the permeability of the comedonal wall to inflammatory mediators. Moreover, linoleic acid activates PPARδ, which is believed to regulate multiple functions of sebocytes, including maturation and sebum production (Citation74). Additionally, PPARγ regulates sebocyte differentiation, sebum production, and anti-inflammatory processes (Citation74–76). Arachidonic acid, an activator of PPARγ, contributes to the upregulation of inflammatory mediators, including leukotriene B4 and IL-6 (Citation77), and increases sebum production in part via PPARγ-dependent signaling pathways (Citation78).
The fatty acid desaturase-2 enzyme (also known as Δ6 desaturase) present in lipid-producing differentiated sebocytes converts palmitic acid (C16:0, a saturated fatty acid) to sapienic acid (C16:1Δ6, an unsaturated fatty acid) (Citation79). However, the role of sapienic acid in AV pathophysiology is controversial, with some studies demonstrating that an abundance of this fatty acid was related to increased sebum production (Citation14,Citation15), whereas other studies showed it to inhibit bacteria commonly associated with AV (Citation80,Citation81).
Another hallmark of sebum alterations in AV is an increase in levels of squalene and squalene peroxide (Citation14,Citation82), which contributes to comedone formation and the development of inflammatory AV (Citation83). Beyond sebum quantity and quality, the location of sebum production and patient characteristics can also influence the development of AV (Citation84–87).
4. Factors influencing sebum quality and quantity
Facial sebum secretion has subregional variations that contribute to clinical differences in AV lesions () (Citation84). In both patients with and without AV, sebum levels are highest on the nose, followed by the forehead, chin, and cheeks (Citation88). Accordingly, the T-zone (comprising the forehead, nose, and chin) is considered a high sebum-secreting zone, whereas the O-zone (encompassing the perioral area) and U-zone (comprising both cheeks) are considered to be moderate-to-high and low sebum-secreting zones, respectively (Citation88,Citation89). T-zone, U-zone, and overall face sebum secretions are higher in patients with AV compared with those without AV (Citation90). More recently, the impact of higher temperatures on sebum production was demonstrated in patients with “maskne,” a form of acne commonly found on the perioral region that is associated with prolonged face mask use (Citation85). Further, the higher skin temperature on the forehead and cheeks compared with other regions of the face could contribute to higher sebum levels in the T-zone (Citation91,Citation92).
Figure 2. Sebum production trends by facial region. Sebum levels are highest on the nose, followed by the forehead and chin (comprising the T-zone), and are lowest on the cheeks (comprising the U-zone), regardless of the presence of acne lesions (Citation86,Citation87). Differences in sebum production have also been noted based on patient age and sex (Citation85,Citation87).
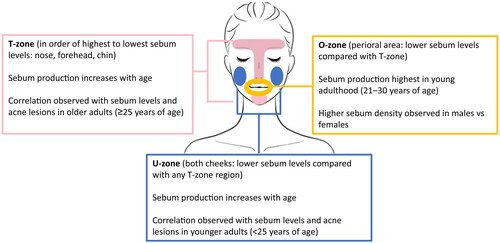
In patients with AV, the level of sebum is significantly higher on the nose compared with the forehead, though this trend has not been observed in patients without AV (Citation88). Further, patients with AV have higher sebum levels on the nose compared with patients without AV, leading to higher sebum levels in the T-zone but not the U-zone (Citation88). This may be attributed, in part, to a lower pH in the T-zone compared with the U-zone in both patients with and without AV (Citation88). The importance of sebum location in AV pathophysiology is also exemplified by the observation that the scalp is a region of high sebum secretion, although this is rarely a site of AV manifestation (Citation90).
Patient age and sex/gender also impact these regional differences in sebum density (Citation89). Sebum excretion rates on the forehead and cheeks decrease with age (Citation86). In younger patients (<25 years of age), sebum levels correlated with AV lesions in the U-zone, whereas this correlation was observed in the T-zone in older patients (≥25 years of age) (Citation87). Sebum density decreases in the NT-zone (comprising the traditional T-zone minus the area around the mouth), U-zone, and whole face with increasing age (Citation89). However, sebum density in the O-zone is highest in young adults aged 21–30 years (Citation89). Further, males have higher sebum density in the O-zone compared with females, and AV in males appears to be more influenced by sebum levels (Citation87,Citation89). Patient characteristics also affect the recommendations for AV therapies that demonstrate an impact on sebum, which include combined oral contraceptives (COCs), oral spironolactone, oral isotretinoin, and topical clascoterone (cortexolone 17α-propionate; ) (Citation6,Citation7,Citation17,Citation99).
Table 1. Overview of pharmacologic therapies for patients with acne and the impact on sebaceous immunobiology.
5. Sebum-targeting therapies
5.1. Standard of care treatments
The use of COCs and spironolactone is limited to female patients with moderate or moderate-to-severe AV, and COCs are further restricted to patients who want or agree to avoid pregnancy (Citation1,Citation17–19,Citation93,Citation94). COCs contain estrogen (primarily ethinyl estradiol [EE]) and progestin components, which together inhibit ovarian androgen production and reduce circulating testosterone levels to reduce sebum production (Citation7). Earlier COC formulations contained higher doses of estrogen, which could directly suppress sebum production (Citation7). In the US, 4 COCs are indicated for the treatment of acne: EE/norgestimate, EE/norethindrone acetate, EE/drospirenone, and EE/drospirenone/levomefolic acid (Citation18,Citation19,Citation93,Citation94). Oral spironolactone is an antiandrogen therapy used off label that blocks sebum production (Citation7,Citation100). The efficacy of topical spironolactone for AV has not been demonstrated in clinical trials, and it is not currently approved for use in patients with AV (Citation101).
Isotretinoin is an oral retinoid that targets all 4 factors involved in AV pathophysiology (Citation6). Through modulation of gene expression, isotretinoin decreases sebocyte proliferation and sebum production and inhibits terminal differentiation of sebocytes, leading to a reduction in sebaceous gland size (Citation7,Citation52). Isotretinoin can be used in male and female patients with severe AV but must be avoided in pregnancy due to the risk of teratogenicity (Citation21).
Clascoterone is the first topical antiandrogen therapy approved for the treatment of AV in both adolescents and adults (i.e., patients ≥12 years of age) with moderate-to-severe facial AV (Citation6,Citation96). Clascoterone modulates sebaceous gland activity through the inhibition of androgen receptors in sebocytes (Citation97). There are no contraindications to clascoterone use, though safety during pregnancy and lactation is unknown (Citation96).
5.2. Emerging pharmacologic treatments and natural products
In recent years, emerging pharmacologic agents evaluated in AV clinical trials have focused on targeting sebocyte function (Citation102). N-acetyl-GED-0507-34-LEVO is a PPARγ inhibitor with sebosuppressive effects in vitro shown to reduce AV severity and total lesion count compared with placebo in a Phase 2b trial including patients with moderate-to-severe AV (Citation103). Additionally, olumacostat glasaretil is a topical small-molecule inhibitor of acetyl coenzyme A carboxylase, which is the rate-limiting enzyme in sebum lipid production (Citation102,Citation104). However, with only modest activity against acne lesions compared with vehicle in a Phase 2a clinical trial, the development of olumacostat glasaretil was halted (Citation102,Citation104).
Some nonpharmacologic or natural products have been shown to impact sebum production or comedogenic potential and represent viable alternative options over conventional therapies for patients with AV based on their history of use, patient tolerance, and safety profiles (Citation52,Citation105). In an open-label randomized clinical trial including adults with moderate-to-severe acne, lactosporin 2% cream, a topical postbiotic targeting both C. acnes and the 5-alpha reductase enzyme involved in androgen production, significantly reduced sebum production compared with benzoyl peroxide 2.5% gel (Citation105). Further, dietary supplementation with omega-3 fatty acids, shown to liquefy sebum and reduce obstruction of the sebaceous gland, can improve acne lesions in patients with mild-to-moderate acne (Citation23,Citation106). Supplementation with vitamins B, D, or E; selenium; or lactoferrin may also reduce sebum production (Citation23).
6. Conclusions
Although AV is highly prevalent across the globe, it is still not considered curable (Citation52). An improved understanding of the mechanisms regulating sebum dysregulation in AV is needed to innovate therapeutic approaches for patients (Citation30). Excess sebum production and changes in sebum composition are necessary early steps in AV pathophysiology (Citation107). Alterations in sebum composition affect sebocyte proliferation, differentiation, and sebum production, as well as hyperkeratinization and inflammation within the sebaceous gland (Citation22,Citation25,Citation47). Changes in sebum production vary by facial region as well as other factors, such as patient sex and age (Citation84–87). There is an unmet need for agents that target excessive sebum production and alterations in sebum compositions that can be safely used in a variety of patients, including male patients and patients who are or may become pregnant (Citation6,Citation22). Topical antiandrogens devoid of systemic side effects, such as clascoterone, address many of these unmet needs for a more diverse group of patients, including males (Citation6).
Acknowledgments
Manuscript preparation and editorial support were provided by AlphaBioCom, a Red Nucleus company. This support was funded by Sun Pharma.
Disclosure statement
JDR has served as a research investigator, consultant, and/or speaker for Allergan, Almirall, Amgen (Celgene), Arcutis, Bausch Health (Ortho Dermatologics), Bayer Healthcare Pharmaceuticals, Beiersdorf, Biorasi, Bristol Myers Squibb, Cassiopea, Cutera, Dermavant Sciences, Dr Reddy, Eli Lilly, EPI Health, Evommune, Ferndale, Galderma, JEM Health, Johnson & Johnson, Journey, LEO Pharma, L’Oréal, Mayne Pharma, Novan, Sebacia, Sol-Gel, Sun Pharma, Strata, and Vyne. LK has served as an investigator, speaker, advisory board member, or consultant for 3 M, Abbott, Aclaris Therapeutics, Allergan, Amgen, Anacor Pharmaceuticals, Assos Pharmaceuticals, Astellas Pharma US, Asubio Pharma, Bayer Healthcare Pharmaceuticals, Berlex Laboratories (Bayer Healthcare Pharmaceuticals), Biogen, BioLife, Biopelle, Blue Willow Biologics, Boehringer Ingelheim, Breckenridge Pharmaceutical, Celgene Corporation, Centocor, ColBar LifeScience, CollaGenex Pharmaceuticals, Combimatrix Molecular Diagnostics, Connetics Corporation, Coria Laboratories, Dermik Laboratories, Dermira, Dow Pharmaceutical Sciences, DUSA Pharmaceuticals, Eli Lilly, Embil Pharmaceutical, EOS Pharmaceutical, Ferndale Pharma Group, Galderma, Genentech, GSK, Healthpoint, Idera Pharmaceuticals, Innocutis Medical, Innovail, Johnson & Johnson, Laboratory Skin Care, LEO Pharma, L’Oréal, Maruho, Medical International Technologies, Medicis Pharmaceutical, Merck, Merz Pharma, Novartis AG, Noven Pharmaceuticals, Nucryst Pharmaceuticals, Obagi Medical Products, Ortho Neutrogena, Pediapharma, Pfizer, PharmaDerm, Promius Pharma, PuraCap Pharmaceutical, QLT, Quatrix, Quinnova Pharmaceuticals, Serono (Merck-Serono International), SkinMedica, Stiefel Laboratories, Sun Pharma, Taro Pharmaceutical Industries, TolerRx, Triax Pharmaceuticals, UCB, Valeant Pharmaceuticals North America, Warner Chilcott, XenoPort, and ZAGE.
Data availability statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
Additional information
Funding
References
- Costa CS, Bagatin E, Yang Z, et al. Systemic pharmacological treatments for acne: an overview of systematic reviews (protocol). Cochrane Database Syst Rev. 2021;2021(11):1.
- Leung AK, Barankin B, Lam JM, et al. Dermatology: how to manage acne vulgaris. Drugs Context. 2021;10:2021-8-6. doi: 10.7573/dic.2021-8-6.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2163–8. doi: 10.1016/S0140-6736(12)61729-2.
- Lynn DD, Umari T, Dunnick CA, et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25. doi: 10.2147/AHMT.S55832.
- Layton AM, Thiboutot D, Tan J. Reviewing the global burden of acne: how could we improve care to reduce the burden? Br J Dermatol. 2021;184(2):219–225. doi: 10.1111/bjd.19477.
- Baldwin H, Farberg A, Frey C, et al. Unmet needs in the management of acne vulgaris: a consensus statement. J Drugs Dermatol. 2023;22(6):582–587. doi: 10.36849/JDD.7587.
- Zouboulis CC, Piquero-Martin J. Update and future of systemic acne treatment. Dermatology. 2003;206(1):37–53. doi: 10.1159/000067821.
- Del Rosso JQ, Kircik LH, Stein Gold L, et al. Androgens, androgen receptors, and the skin: from the laboratory to the clinic with emphasis on clinical and therapeutic implications. J Drugs Dermatol. 2020;19(3):30–35.
- Elsaie ML. Hormonal treatment of acne vulgaris: an update. Clin Cosmet Investig Dermatol. 2016;9:241–248. doi: 10.2147/CCID.S114830.
- Dudhat S, Singh P, Pimple P. Insights of lipid vesicular and particulate carrier mediated approach for acne management. Curr Drug Deliv. 2022;20(1):57–74. doi: 10.2174/1567201819666220524154448.
- Cunliffe WJ. Androgen abnormalities in acne subjects. In: Acne. London (UK): Martin Dunitz Ltd; 1989.
- Nelson AT. Sebum. In: Shalita AR, Del Rosso JQ, Webster GF, editors. Acne vulgaris. London (UK): Informa Healthcare; 2011.
- Camera E, Ludovici M, Tortorella S, et al. Use of lipidomics to investigate sebum dysfunction in juvenile acne. J Lipid Res. 2016;57(6):1051–1058. doi: 10.1194/jlr.M067942.
- Pappas A, Johnsen S, Liu JC, et al. Sebum analysis of individuals with and without acne. Dermatoendocrinol. 2009;1(3):157–161. doi: 10.4161/derm.1.3.8473.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50(1):41–52. doi: 10.1016/j.jdermsci.2007.11.005.
- Cao K, Liu Y, Liang N, et al. Fatty acid profiling in facial sebum and erythrocytes from adult patients with moderate acne. Front Physiol. 2022;13:921866. doi: 10.3389/fphys.2022.921866.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973 e33. doi: 10.1016/j.jaad.2015.12.037.
- YAZ® (Drospirenone and ethinyl estradiol) tablets [prescribing information]. Whippany (NJ): Bayer HealthCare Pharmaceuticals Inc; 2023.
- BEYAZ® (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets), for oral use [prescribing information]. Whippany (NJ): Bayer HealthCare Pharmaceuticals Inc.; 2023.
- Aldactone® (spironolactone) tablets, for oral use [prescribing information]. New York (NY): Pfizer Labs; 2022.
- ACCUTANE® (isotretinoin) [prescribing information]. Nutley (NJ): Roche Laboratories Inc.; 2008.
- Zouboulis CC, Coenye T, He L, et al. Sebaceous immunobiology – skin homeostasis, pathophysiology, coordination of innate immunity and inflammatory response and disease associations. Front Immunol. 2022;13:1029818. doi: 10.3389/fimmu.2022.1029818.
- Podgórska A, Puścion-Jakubik A, Markiewicz-Żukowska R, et al. Acne vulgaris and intake of selected dietary nutrients-a summary of information. Healthcare (Basel). 2021;9(6):668. doi: 10.3390/healthcare9060668.
- Hoover E, Aslam S, Krishnamurthy K. Physiology, sebaceous glands. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- Zouboulis CC. Endocrinology and immunology of acne: two sides of the same coin. Exp Dermatol. 2020;29(9):840–859. doi: 10.1111/exd.14172.
- Plewig G, Kligman AM. Proliferative activity of the sebaceous glands of the aged. J Invest Dermatol. 1978;70(6):314–317. doi: 10.1111/1523-1747.ep12543478.
- Pochi PE, Strauss JS, Downing DT. Age-related changes in sebaceous gland activity. J Invest Dermatol. 1979;73(1):108–111. doi: 10.1111/1523-1747.ep12532792.
- Yamamoto A, Ito M. Sebaceous gland activity and urinary androgen levels in children. J Dermatol Sci. 1992;4(2):98–104. doi: 10.1016/0923-1811(92)90066-k.
- Pochi PE, Strauss JS, Downing DT. Skin surface lipid composition, acne, pubertal development, and urinary excretion of testosterone and 17-ketosteroids in children. J Invest Dermatol. 1977;69(5):485–489. doi: 10.1111/1523-1747.ep12511753.
- Ottaviani M, Camera E, Picardo M. Lipid mediators in acne. Mediators Inflamm. 2010;2010:858176. doi: 10.1155/2010/858176.
- Akamatsu H, Zouboulis CC, Orfanos CE. Control of human sebocyte proliferation in vitro by testosterone and 5-alpha-dihydrotestosterone is dependent on the localization of the sebaceous glands. J Invest Dermatol. 1992;99(4):509–511. doi: 10.1111/1523-1747.ep12616181.
- Sato T, Imai N, Akimoto N, et al. Epidermal growth factor and 1alpha,25-dihydroxyvitamin D3 suppress lipogenesis in hamster sebaceous gland cells in vitro. J Invest Dermatol. 2001;117(4):965–970. doi: 10.1046/j.0022-202x.2001.01516.x.
- Tsukada M, Schröder M, Roos TC, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115(2):321–327. doi: 10.1046/j.1523-1747.2000.00066.x.
- Hong I, Lee MH, Na TY, et al. LXRalpha enhances lipid synthesis in SZ95 sebocytes. J Invest Dermatol. 2008;128(5):1266–1272. doi: 10.1038/sj.jid.5701134.
- Rosenfield RL, Deplewski D, Kentsis A, et al. Mechanisms of androgen induction of sebocyte differentiation. Dermatology. 1998;196(1):43–46. doi: 10.1159/000017864.
- Fischer H, Fumicz J, Rossiter H, et al. Holocrine secretion of sebum is a unique DNASE2-dependent mode of programmed cell death. J Invest Dermatol. 2017;137(3):587–594. doi: 10.1016/j.jid.2016.10.017.
- Jung Y, Tam J, Ray Jalian H, et al. Longitudinal, 3D in vivo imaging of sebaceous glands by coherent anti-stokes Raman scattering microscopy: normal function and response to cryotherapy. J Invest Dermatol. 2015;135(1):39–44. doi: 10.1038/jid.2014.293.
- Plewig G, Christophers E. Renewal rate of human sebaceous glands. Acta Derm Venereol. 1974;54(3):177–182. doi: 10.2340/0001555554177182.
- Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004;17(Suppl 1):6–15. doi: 10.1111/j.1396-0296.2004.04s1001.x.
- Greene RS, Downing DT, Pochi PE, et al. Anatomical variation in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970;54(3):240–247. doi: 10.1111/1523-1747.ep12280318.
- Cunliffe WJ. Biochemistry of the pilosebaceous unit. In: Acne. London (UK): Martin Dunitz Ltd; 1989.
- Cunliffe WJ. Sebaceous gland physiology. In: Acne. London (UK): Martin Dunitz Ltd; 1989.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 Suppl):S109–S115.
- Kim YJ, Lee SB, Lee HB. Oleic acid enhances keratinocytes differentiation via the upregulation of miR-203 in human epidermal keratinocytes. J Cosmet Dermatol. 2019;18(1):383–389. doi: 10.1111/jocd.12543.
- Lovászi M, Mattii M, Eyerich K, et al. Sebum lipids influence macrophage polarization and activation. Br J Dermatol. 2017;177(6):1671–1682. doi: 10.1111/bjd.15754.
- Nakatsuji T, Kao MC, Zhang L, et al. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol. 2010;130(4):985–994. doi: 10.1038/jid.2009.384.
- Li X, He C, Chen Z, et al. A review of the role of sebum in the mechanism of acne pathogenesis. J Cosmet Dermatol. 2017;16(2):168–173. doi: 10.1111/jocd.12345.
- Makrantonaki E, Ganceviciene R, Zouboulis C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermatoendocrinol. 2011;3(1):41–49. doi: 10.4161/derm.3.1.13900.
- Kligman AM, Wheatley VR, Mills OH. Comedogenicity of human sebum. Arch Dermatol. 1970;102(3):267–275. doi: 10.1001/archderm.1970.04000090029005.
- Kligman AM, Katz AG. Pathogenesis of acne vulgaris. I. Comedogenic properties of human sebum in external ear canal of the rabbit. Arch Dermatol. 1968;98(1):53–57. doi: 10.1001/archderm.1968.01610130059013.
- Li WH, Zhang Q, Flach CR, et al. In vitro modeling of unsaturated free fatty acid-mediated tissue impairments seen in acne lesions. Arch Dermatol Res. 2017;309(7):529–540. doi: 10.1007/s00403-017-1747-y.
- Mohiuddin AK. A comprehensive review of acne vulgaris. CRDOA. 2019;6(2):1–34. doi: 10.15226/2378-1726/6/2/00186.
- Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–1192. doi: 10.1126/science.1171700.
- Borrel V, Gannesen AV, Barreau M, et al. Adaptation of acneic and non acneic strains of Cutibacterium acnes to sebum-like environment. MicrobiologyOpen. 2019;8(9):e00841. doi: 10.1002/mbo3.841.
- Josse G, Mias C, Le Digabel J, et al. High bacterial colonization and lipase activity in microcomedones. Exp Dermatol. 2020;29(2):168–176. doi: 10.1111/exd.14069.
- Clayton RW, Langan EA, Ansell DM, et al. Neuroendocrinology and neurobiology of sebaceous glands. Biol Rev Camb Philos Soc. 2020;95(3):592–624. doi: 10.1111/brv.12579.
- Harris HH, Downing DT, Stewart ME, et al. Sustainable rates of sebum secretion in acne patients and matched normal control subjects. J Am Acad Dermatol. 1983;8(2):200–203. doi: 10.1016/s0190-9622(83)70023-x.
- Piérard GE, Piérard-Franchimont C, Lê T. Seborrhoea in acne-prone and acne-free patients. Dermatologica. 1987;175(1):5–9. doi: 10.1159/000248774.
- Piérard-Franchimont C, Piérard GE, Saint-Léger D, et al. Comparison of the kinetics of sebum secretion in young women with and without acne. Dermatologica. 1991;183(2):120–122. doi: 10.1159/000247650.
- Çölgeçen E, Özyurt K, Ferahbaş Kesikoğlu A. Ferahbas kesikoglu A. The effect of systemic isotretinoin treatment on skin biophysicalparameters among patients with acne vulgaris. Turk J Med Sci. 2016;46(6):1641–1644. doi: 10.3906/sag-1507-31.
- Hughes BR, Cunliffe WJ. A prospective study of the effect of isotretinoin on the follicular reservoir and sustainable sebum excretion rate in patients with acne. Arch Dermatol. 1994;130(3):315–318. doi: 10.1001/archderm.1994.01690030047007.
- Andreadi A, Muscoli S, Tajmir R, et al. Insulin resistance and acne: the role of metformin as alternative therapy in men. Pharmaceuticals (Basel). 2022;16(1):27. doi: 10.3390/ph16010027.
- Hay JB, Hodgins MB. Metabolism of androgens by human skin in acne. Br J Dermatol. 1974;91(2):123–133. doi: 10.1111/j.1365-2133.1974.tb15857.x.
- Hay JB, Hodgins MB. Distribution of androgen metabolizing enzymes in isolated tissues of human forehead and axillary skin. J Endocrinol. 1978;79(1):29–39. doi: 10.1677/joe.0.0790029.
- Tan MH, Li J, Xu HE, et al. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36(1):3–23. doi: 10.1038/aps.2014.18.
- Burkhart CG, Burkhart CN. Expanding the microcomedone theory and acne therapeutics: propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J Am Acad Dermatol. 2007;57(4):722–724. doi: 10.1016/j.jaad.2007.05.013.
- Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Res. 2018;7:1953. doi: 10.12688/f1000research.15659.1.
- Krause K, Schnitger A, Fimmel S, et al. Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm Metab Res. 2007;39(2):166–170. doi: 10.1055/s-2007-961811.
- Wang Y, Hata TR, Tong YL, et al. The anti-inflammatory activities of Propionibacterium acnes camp factor-targeted acne vaccines. J Invest Dermatol. 2018;138(11):2355–2364. doi: 10.1016/j.jid.2018.05.032.
- Stewart ME, Grahek MO, Cambier LS, et al. Dilutional effect of increased sebaceous gland activity on the proportion of linoleic acid in sebaceous wax esters and in epidermal acylceramides. J Invest Dermatol. 1986;87(6):733–736. doi: 10.1111/1523-1747.ep12456856.
- Perisho K, Wertz PW, Madison KC, et al. Fatty acids of acylceramides from comedones and from the skin surface of acne patients and control subjects. J Invest Dermatol. 1988;90(3):350–353. doi: 10.1111/1523-1747.ep12456327.
- Elias PM, Brown BE, Ziboh VA. The permeability barrier in essential fatty acid deficiency: evidence for a direct role for linoleic acid in barrier function. J Invest Dermatol. 1980;74(4):230–233. doi: 10.1111/1523-1747.ep12541775.
- Simard M, Tremblay A, Morin S, et al. α-Linolenic acid and linoleic acid modulate the lipidome and the skin barrier of a tissue-engineered skin model. Acta Biomater. 2022;140:261–274. doi: 10.1016/j.actbio.2021.11.021.
- Rosenfield RL, Kentsis A, Deplewski D, et al. Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors. J Invest Dermatol. 1999;112(2):226–232. doi: 10.1046/j.1523-1747.1999.00487.x.
- Trivedi NR, Cong Z, Nelson AM, et al. Peroxisome proliferator-activated receptors increase human sebum production. J Invest Dermatol. 2006;126(9):2002–2009. doi: 10.1038/sj.jid.5700336.
- Mastrofrancesco A, Ottaviani M, Cardinali G, et al. Pharmacological PPARγ modulation regulates sebogenesis and inflammation in SZ95 human sebocytes. Biochem Pharmacol. 2017;138:96–106. doi: 10.1016/j.bcp.2017.04.030.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl). 2006;84(1):75–87. doi: 10.1007/s00109-005-0715-8.
- Dozsa A, Dezso B, Toth BI, et al. PPARγ-mediated and arachidonic acid-dependent signaling is involved in differentiation and lipid production of human sebocytes. J Invest Dermatol. 2014;134(4):910–920. doi: 10.1038/jid.2013.413.
- Ge L, Gordon JS, Hsuan C, et al. Identification of the delta-6 desaturase of human sebaceous glands: expression and enzyme activity. J Invest Dermatol. 2003;120(5):707–714. doi: 10.1046/j.1523-1747.2003.12123.x.
- Drake DR, Brogden KA, Dawson DV, et al. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49(1):4–11. doi: 10.1194/jlr.R700016-JLR200.
- Fischer CL, Drake DR, Dawson DV, et al. Antibacterial activity of sphingoid bases and fatty acids against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2012;56(3):1157–1161. doi: 10.1128/AAC.05151-11.
- Okoro OE, Adenle A, Ludovici M, et al. Lipidomics of facial sebum in the comparison between acne and non-acne adolescents with dark skin. Sci Rep. 2021;11(1):16591. doi: 10.1038/s41598-021-96043-x.
- Ottaviani M, Alestas T, Flori E, et al. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in acne vulgaris. J Invest Dermatol. 2006;126(11):2430–2437. doi: 10.1038/sj.jid.5700434.
- Youn SW. The role of facial sebum secretion in acne pathogenesis: facts and controversies. Clin Dermatol. 2010;28(1):8–11. doi: 10.1016/j.clindermatol.2009.03.011.
- Spigariolo CB, Giacalone S, Nazzaro G. Maskne: the epidemic within the pandemic: from diagnosis to therapy. J Clin Med. 2022;11(3):618. doi: 10.3390/jcm11030618.
- Park SG, Kim YD, Kim JJ, et al. Two possible classifications of facial skin type by two parameters in korean women: sebum excretion rate (SER) and skin surface relief (SSR). Skin Res Technol. 1999;5(3):189–194. doi: 10.1111/j.1600-0846.1999.tb00130.x.
- Choi CW, Choi JW, Park KC, et al. Facial sebum affects the development of acne, especially the distribution of inflammatory acne. J Eur Acad Dermatol Venereol. 2013;27(3):301–306. doi: 10.1111/j.1468-3083.2011.04384.x.
- Kim MK, Choi SY, Byun HJ, et al. Comparison of sebum secretion, skin type, pH in humans with and without acne. Arch Dermatol Res. 2006;298(3):113–119. doi: 10.1007/s00403-006-0666-0.
- Youn SH, Choi CW, Choi JW, et al. Novel facial cosmetic area ‘O zone’ shows unique characteristics in sebum excretion and acne lesion distribution. Skin Res Technol. 2014;20(2):164–169. doi: 10.1111/srt.12101.
- Youn SW, Park ES, Lee DH, et al. Does facial sebum excretion really affect the development of acne? Br J Dermatol. 2005;153(5):919–924. doi: 10.1111/j.1365-2133.2005.06794.x.
- Lopez S, Le Fur I, Morizot F, et al. Transepidermal water loss, temperature and sebum levels on women’s facial skin follow characteristic patterns. Skin Res Technol. 2000;6(1):31–36. doi: 10.1034/j.1600-0846.2000.006001031.x.
- Le Fur I, Lopez S, Morizot F, et al. Comparison of cheek and forehead regions by bioengineering methods in women with different self-reported “cosmetic skin types”. Skin Res Technol. 2006;5(3):182–188. doi: 10.1111/j.1600-0846.1999.tb00129.x.
- ORTHO TRI-CYCLEN® tablets (norgestimate/ethinyl estradiol) [prescribing information]. Tulsa (OK): Physicians Total Care, Inc.; 2012.
- ESTROSTEP® Fe (norethindrone acetate and ethinyl estradiol tablets, USP and ferrous fumarate tablets) [prescribing information]. Irvine (CA): Allergan USA, Inc.; 2017.
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169–191. doi: 10.1007/s40257-016-0245-x.
- WINLEVI® (clascoterone) cream, for topical use [prescribing information]. Cranbury (NJ): Sun Pharmaceutical Industries, Inc.; 2022.
- Rosette C, Rosette N, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is an androgen receptor antagonist in dermal papilla cells in vitro. J Drugs Dermatol. 2019;18(2):197–201.
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18(5):412–418.
- Layton AM, Alexis A, Baldwin H, et al. The personalized acne treatment tool – recommendations to facilitate a patient-centered approach to acne management from the personalizing acne: consensus of experts. JAAD Int. 2023;12:60–69. doi: 10.1016/j.jdin.2023.03.013.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther. 2021;11(1):79–91. doi: 10.1007/s13555-020-00481-w.
- Afzali BM, Yaghoobi E, Yaghoobi R, et al. Comparison of the efficacy of 5% topical spironolactone gel and placebo in the treatment of mild and moderate acne vulgaris: a randomized controlled trial. J Dermatolog Treat. 2012;23(1):21–25. doi: 10.3109/09546634.2010.488260.
- Bhatt S, Kothari R, Tripathy DM, et al. Emerging drugs for the treatment of acne: a review of phase 2 & 3 trials. Expert Opin Emerg Drugs. 2022;27(3):241–261. doi: 10.1080/14728214.2022.2110239.
- Picardo M, Cardinali C, La Placa M, et al. Efficacy and safety of N-acetyl-GED-0507-34-LEVO gel in patients with moderate-to severe facial acne vulgaris: a phase IIb randomized double-blind, vehicle-controlled trial. Br J Dermatol. 2022;187(4):507–514. doi: 10.1111/bjd.21663.
- Bissonnette R, Poulin Y, Drew J, et al. Olumacostat glasaretil, a novel topical sebum inhibitor, in the treatment of acne vulgaris: a phase IIa, multicenter, randomized, vehicle-controlled study. J Am Acad Dermatol. 2017;76(1):33–39. doi: 10.1016/j.jaad.2016.08.053.
- Majeed M, Majeed S, Nagabhushanam K, et al. Novel topical application of a postbiotic, lactosporin®, in mild to moderate acne: a randomized, comparative clinical study to evaluate its efficacy, tolerability and safety. Cosmetics. 2020;7(3):70. doi: 10.3390/cosmetics7030070.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94(5):521–525. doi: 10.2340/00015555-1802.
- Leyden JJ. A review of the use of combination therapies for the treatment of acne vulgaris. J Am Acad Dermatol. 2003;49(3 Suppl):S200–S210. doi: 10.1067/s0190-9622(03)01154-x.

