Abstract
Background
With advent of newer treatments for psoriasis, real-world use of biologics in Japan is evolving.
Methods
This retrospective study utilized data from patients with ≥1 psoriasis-related biologic claims record between January 2016 and December 2020 in Japan to evaluate treatment patterns, healthcare resource utilization (HCRU), and associated costs. Data were analyzed using descriptive statistics.
Results
Of 1,614 eligible patients, 72.5% were male, 29.2% had comorbid hypertension and 26.6% had comorbid cardiovascular disease. Interleukin (IL)-17 and tumor necrosis factor alpha (TNFα) inhibitors were commonly prescribed across lines of treatment, while IL-23 inhibitors were most considered for switches (92% of switches were from IL-12/23/IL-17/TNFα inhibitors). The overall mean adherence rate for all classes was 80.1%, but adherence varied across biologics. Infliximab and IL-23 inhibitor users exhibited optimal medical possession ratios, reflecting the best adherence rates. Overall HCRU (visits/patient-year) was 9.05 for outpatient visits, 0.09 for inpatient hospitalization, and 0.5 for psoriasis-related phototherapy. HCRU associated with hospitalization was slightly higher for bio-experienced patients and so was the overall costs per patient-year relative to bio-naïve patients.
Conclusion
Variable adherence rates observed suggest the need for improvement in treatment management with different biologics. Bio-experienced patients burdened by disease progression and treatment switches may result in increased HCRU.
Introduction
Psoriasis is a chronic, autoimmune, inflammatory skin disease commonly associated with significant comorbidities, including hypertension, diabetes mellitus, dyslipidemia, hyperuricemia, and cardiovascular diseases (Citation1–4). These conditions may cause high disease burden and mortality in patients (Citation4–6). The global incidence of psoriasis was about 4.6 million cases in 2019 and the global prevalence was 2%-3% (Citation6). The estimated prevalence of psoriasis in Japan reported in previous studies was 0.34% to 0.44% in 2021 (Citation1). However, the prevalence of psoriasis in Japan is expected to increase annually, due to lifestyle-related risk factors, such as obesity and smoking (Citation1,Citation4).
Mild to moderate forms of psoriasis are typically treated with topical therapies (corticosteroids, vitamin D3 analogues, calcipotriol + steroid combination treatments) while more severe forms are treated with systemic therapies (oral and injectable: methotrexate, cyclosporine, biologics) and non-pharmaceutical therapy (e.g. phototherapy) (Citation7). In Japan, biologic treatments in the tumor necrosis factor alpha (TNFα) class, namely infliximab (administered by intravenous infusion), and adalimumab, were approved in 2010. Ustekinumab the only biologic in the interleukin (IL)-12/23 class, was first approved in 2011. Biologics from the IL-17 (secukinumab [2015], ixekinumab [2016], brodalumab [2016]) and IL-23 inhibitor classes (guselkumab [2018], risankizumab [2019]) were approved after 2014; and certolizumab pegol (2019), another TNFα inhibitor, was recently approved for treating both psoriasis and psoriatic arthritis (Citation7). These biologics inhibit the action of specific mediators of immune-related pathways that are involved in triggering the signs and symptoms of psoriasis (Citation8,Citation9).
Although the number of treatment options for patients with moderate to severe psoriasis has increased in the past few years, real-world clinical data are limited in Japan (Citation10). The evolving use of established biologics and the availability of newer treatment options has led to a dynamic treatment environment. This ultimately has an impact on treatment patterns and adherence over time (Citation11). Understanding the framework and patterns of biologic treatment usage could support optimizing therapeutic approaches with the aim of improving treatment outcomes and patient satisfaction (Citation10).
Several studies have analyzed the use of biologic treatments and healthcare resource utilization (HCRU) in patients with psoriasis in the United States and in many European countries (Citation12–20). However, there are few studies available using data from Japan (Citation21–23); further, prior studies may not capture the overall scenario of treatment patterns and adherence in patients with psoriasis in Japan (Citation24). Establishing a cohesive body of evidence inclusive of psoriasis patients receiving different advanced treatments using real-world data is crucial for enabling Japanese patients and healthcare providers to make appropriate treatment choices to manage psoriasis effectively.
Furthermore, there is a need to gain better insight into HCRU and associated direct medical costs, as these data could be indicative of treatment adherence to biologics (Citation25,Citation26). Findings from such studies may also highlight the overall economic impact of the disease burden of biologic-treated psoriasis patients from the perspective of the Japanese healthcare system. This retrospective analysis aimed to evaluate patient characteristics, treatment patterns, treatment adherence to biologic therapies, HCRU, and direct medical costs in patients with psoriasis treated with biologics in a real-world setting in Japan.
Methods
Data source
This study utilized commercially available anonymized insurance claims data from the Japan Medical Data Center (JMDC) database, which included approximately 14 million members and represented approximately 11% of the total Japanese population as of 2022 (Citation27). The anonymized dataset was acquired from JMDC in June 2021.
The JMDC database consists of demographic, pharmacy, and medical claims data, including year of birth, sex, year and month of medical service or prescription provided, diagnosis based on the International Classification of Diseases, 10th Revision (ICD-10) codes (Citation28), drug names and dosages, annual days of therapy, inpatient or outpatient care, size and type of hospitals where patients received care, type of claims, and annual company health checkup data.
Study design
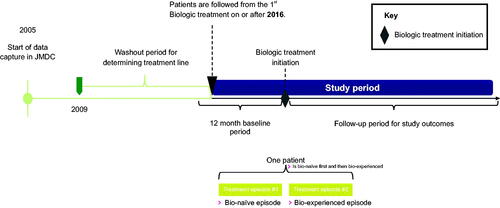
This was an observational, retrospective study, which followed patients longitudinally. Patients diagnosed with either psoriasis only or both psoriasis and psoriatic arthritis as recorded in the JMDC database from 1st January 2005 or later were selected for the study. Insurance claims records were selected from a prespecified study period ranging from 1st January 2016 to 31st December 2020, during which patients’ biologic treatment records were followed longitudinally. The index date was defined as the date of the first biologic psoriasis treatment recorded by an insurance claim within the study period. For each patient, two separate time frames were considered: the baseline period (defined as the 12-month period prior to the index date) and the follow-up period (defined as the period from the index date until the end of data capture, death, or loss to follow-up, whichever came first). Patients’ history of comorbidities, as well as Charlson Comorbidity Index (CCI) (Citation29) scores, and concomitant medications were evaluated during the baseline period. Comorbidities and concomitant medications were additionally assessed longitudinally during the 12-month period following initiation of biologic treatment (). HCRU was analyzed cross-sectionally at 2020 December 31st and one year prior, i.e. earliest 2020 January 1st, or from index date whichever came later, in order to understand the most recent HCRU for the healthcare system.
Study population
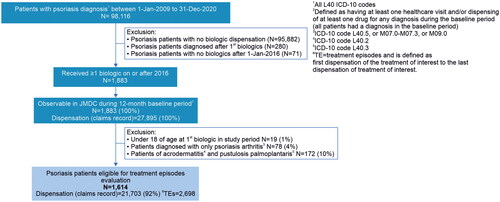
All patients with at least one recorded psoriasis diagnostic code after 1st January 2009 and who were observable in the JMDC database for at least 12 months prior to the index date were assessed for inclusion. Eligible patients were ≥18 years of age at index date and received at least 1 dispensation (claim record) for a biologic treatment during the study period. A list of psoriasis diagnostic ICD-10 codes identified in the JMDC database is presented in Table S1. Patients who received any biologic treatment reimbursed for psoriasis (TNFɑ [adalimumab, infliximab, and certolizumab pegol], IL-12/23 [ustekinumab], IL-17 [secukinumab, brodalumab, and ixekizumab], and IL-23 [guselkumab and risankizumab] inhibitors) were included for evaluation.
Patients were excluded if they had only psoriatic arthritis based on a recorded ICD-10 code of L40.5, or M07.0-M07.3, or M09.0; or no record of a psoriasis diagnostic code (i.e. no record of ICD-10 code L40.0-L40.4, or L40.8, or L40.9); a diagnostic code for acrodermatitis continua (ICD-10: L40.2) and/or pustulosis palmoplantaris (ICD-10: L40.3); and no record of a biologic claim on or after 1st January 2016. Patients diagnosed with psoriasis for the first time after dispensation of the first biologic treatment were also excluded. The patient flow diagram is shown in .
Outcomes/assessments
Treatment patterns
All biologic treatment episodes were longitudinally followed from treatment initiation to discontinuation. Treatment switch was defined as having a claims record for a different biologic after discontinuation of the previous treatment episode. A grace period was defined to account for patients who may not have received the next dose of their prescribed biologic as scheduled, but otherwise continued treatment. If the next claim record for that biologic occurred within the allowable grace period, the treatment episode was assumed to have continued. A grace period of 90 days was used for all biologics with a dosing interval of ≤30 days (as per treatment guidelines), and a grace period of 180 days was used for biologics with a dosing interval of >30 days (Table S2). If the treatment gap exceeded the grace period, the treatment episode was considered to have been discontinued. Resumption of the same biologic after the permitted grace period was considered as a next line of treatment.
Treatment adherence
Treatment adherence was estimated based on the medication possession ratio (MPR), which is defined as the proportion of days during a treatment episode that a patient was in possession of treatment supply. The MPR was calculated as the total number of supply days for all claims of a given biologic during a treatment episode, divided by the number of days in the treatment episode.
HCRU and cost
Data for HCRU and medical costs were calculated cross-sectionally across patient groups. HCRU included the number of outpatient visits, hospitalizations, length of stay and psoriasis-related phototherapy procedures. HCRU and costs were analyzed cross-sectionally by calculating retrospectively for both bio experienced and bio naïve patients. For HCRU, the rate per patient-year for total outpatient visitations, inpatient admissions or psoriasis-related phototherapy was calculated as the sum of each claims record event divided by the total patient-years of follow-up within the 2020 calendar year. Total length of hospital stay per patient-year was calculated as the sum of all hospitalization days divided by the total patient-years of follow-up within the 2020 calendar year. Similarly, overall direct medical costs were calculated as the sum of costs from inpatient and outpatient care, the total cost of psoriasis-related pharmaceutical treatment, and the total cost of psoriasis-related phototherapy procedures divided by the total patient-years of follow-up within the 2020 calendar year.
Statistical analysis
Descriptive statistics were used for all analyses and presented as means (SD), medians and ranges (25th percentile [Q1] and 75th percentile [Q3]), frequency, and percentages. Patient characteristics were analyzed only for the first treatment episode in the study period and reported descriptively using mean, median, and interquartile ranges for continuous variables; and frequency and percentages for categorical variables. The values obtained in all analyses were rounded to a single decimal place.
Treatment adherence was presented as the percentage of treatment episodes with an MPR value of ≥0.80 (80%), with optimal adherence considered to be near a value 1.0 (100%) (Citation30). HCRU and costs were reported as rates per patient–year for the overall analysis population and by prior biologic use subgroups (i.e. bio-experienced and bio-naïve). The costs of outpatient and inpatient visits were assessed separately as points per claim using the diagnosis procedure combination (DPC) variable that specifies the cost of each visit. All data management, calculations, and statistical analyses were performed using the statistical analysis software SAS Version 9.4 (SAS Institute Inc. Cary, NC, USA) and R version 4.2.0.
Ethics approval
All required approvals and permissions for data extraction from the JMDC database and performing pre-specified analyses were obtained. The study was conducted in compliance with all laws and regulations, national guidelines, and other standards regarding the handling of personal information. Informed consent and ethics committee approval were not applicable based on the Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Welfare and Labor.
Results
Patient disposition
Of all patients enrolled in the JMDC database, 98,116 with a confirmed diagnosis of psoriasis (ICD-10 L40) between 2009 and 2020 were identified. Of these, 1,883 patients had at least one record of a biologic treatment claim at the start of the study period (on or after 1st January 2016). After excluding patients who did not meet inclusion criteria; 1,614 patients were retained for evaluation and analysis ().
Patient demographic characteristics
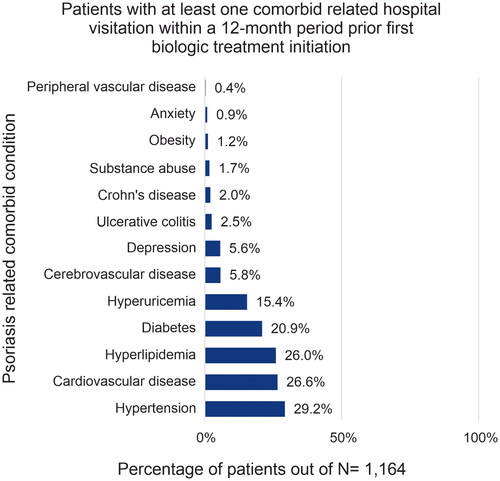
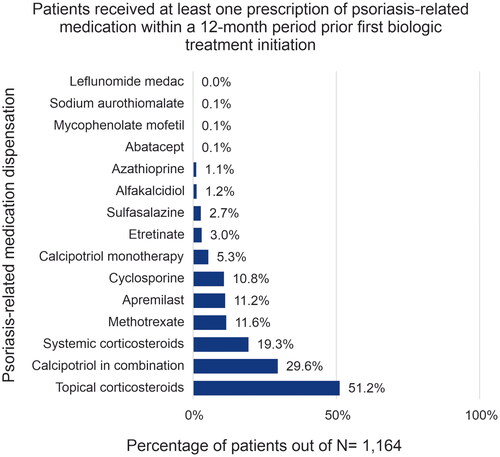
Among the 1,614 patients included, median age was 48.4 years (interquartile range [IQR: 40.6–55.5]), 72.5% of patients were male, and 38.3% had a diagnosis of both psoriasis and psoriatic arthritis (). The demographic characteristics across biologic treatment groups were generally similar. In the TNFα inhibitor group 52.1% (325/624) patients had both psoriasis and psoriatic arthritis whereas 47.9% (299/624) were diagnosed with psoriasis only; in contrast in the IL-12/23 inhibitor group, 19.8% (64/324) patients were co-diagnosed with psoriasis and psoriatic arthritis and 80.2% (260/324) were diagnosed with psoriasis only. Overall, patients initiating biologic treatment had low CCI scores, with a mean score of 0.85. Common comorbidities (>25%) observed include hypertension (29.2%), cardiovascular diseases (26.6%), and hyperlipidemia (26.0%) (), whereas common concomitant medications (>15%) included topical corticosteroids (51.2%), calcipotriol in combination with other topical medications (29.6%), and systemic corticosteroids (19.3%) ().
Table 1. Baseline demographics and characteristics of psoriasis patients who received biologic treatment.
The proportion of patients with at least one claims record for hospital visitation related to comorbidities such as hypertension, hyperuricemia and diabetes slightly increased in the TNFα inhibitor group during the 12-months after initiating treatment. The proportions of patients with at least one cardiovascular disease related hospital visitation claims record in the IL-17 and IL-23 groups showed a slight decrease during the 12-months after initiating treatment [Table S7]. The proportion of patients with at least one prescription for a concomitant medication, such as topical corticosteroids, systemic corticosteroids, methotrexate, and calcipotriol, decreased during the 12-months after initiating IL treatments and increased after initiating TNFα inhibitors in general (Table S8).
Treatment patterns
The patterns of biologic treatment utilization, switching, or resumption are characterized as below.
Treatment sequence
First-line treatment: TNFα (35% [n = 466]) and IL-17 (34% [n = 463]) inhibitors were most commonly used as a first-line biologic treatment (index therapy), followed by IL-12/23 (17% [n = 234]), and IL-23 (14% [n = 185]) inhibitors (). During the study period 38% (n = 178) of TNFα inhibitor users, 38% (n = 89) of IL-12/23 inhibitor users and 36% (n = 168) of IL-17 inhibitor users switched to a second-line biologic compared with 17% (n = 31) of IL-23 inhibitor users ( and Table S9).
Figure 5. Treatment sequences among classes of biologics for psoriasis.
IL, interleukin; TNFα, tumor necrosis factor
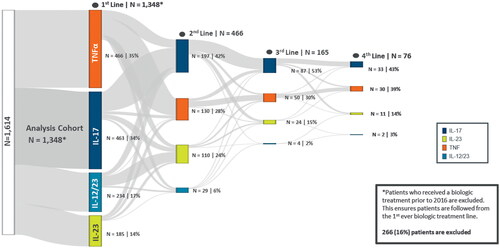
Second-line treatment: IL-17 inhibitors were the most utilized second-line biologic treatment (i.e. switch therapy; 42% [n = 197]), followed by TNFα (28% [n = 130]) and IL-23 (24% [n = 110]) inhibitors. Patterns of treatment switching to a second-line biologic were assessed, and both within- and between-class treatment switches were observed. Among second-line TNFɑ inhibitor users (n = 130), the majority (78% [n = 102]) switched within the class, while 22% switched from other classes (IL-17, 14% [n = 18]; IL-23, 7% [n = 9]; IL-12/23, 1% [n = 1]). Among second-line IL-17 inhibitor users (n = 197), 41% switched from other biologics (TNFɑ inhibitors, 25% [n = 49]; IL-12/23 inhibitor, 10% [n = 19]; IL-23 inhibitors, 6% [n = 12], and 59% (n = 117) switched within IL-17 inhibitor class. Among second-line IL-12/23 inhibitor users (n = 29), 34% (n = 10) patients discontinued and resumed IL-12/23 inhibitor treatment, while the majority (65%) had switched from other classes (TNFɑ inhibitors, 34% [n = 10], IL-17 inhibitors, 28% [n = 8], IL-23 inhibitors, 3% [n = 1]). Of the 110 patients receiving IL-23 inhibitors as second-line treatment, 54% (n = 59) switched from the IL-12/23 inhibitor, 23% (n = 25) switched from IL-17 inhibitors and 15% (n = 17) switched from the TNFɑ inhibitor class, while 8% (n = 9) switched within the IL-23 class. IL-23 inhibitors were most considered for switches (92%) as maximum switches into IL-23 occurred from IL-12/23/IL-17/TNFα inhibitors ( and Table S9). Third and fourth-line treatment distribution was similar to that of second line.
Time to switch and time to resumption of biologic treatment
The overall median time to switching biologic treatment was 9.4 months. The median time to switch was longer for the bio-naïve subgroup (10.9 months) than for the bio-experienced subgroup (8.1 months). The median time to resumption of the same biologic treatment was 9.7 months. Similarly, the time to resuming the same treatment was longer for the bio-naïve subgroup (12.6 months) than for the bio-experienced subgroup (7.5 months) ().
Table 2. Time to switch and resumption of biologics in months.
Treatment adherence
The overall mean adherence rate was 80.1% across identified treatment episodes during the study period. The 1,348 bio-naïve treatment episodes showed a higher mean adherence rate of 82.8%, compared to the 1,350 bio-experienced treatment episodes (77.4%). The MPRs nearest to 100%, defined as optimal adherence, were observed for infliximab (105.7%), guselkumab (100.1%), and ustekinumab (100.1%). The lowest MPRs were observed for patients who received certolizumab pegol (67.5%), brodalumab (56.3%) and adalimumab (54.5%), while the highest MPR recorded was for risankizumab (119.6%) ().
Table 3. Treatment adherence as represented by medication possession ratio (MPR).
HCRU and direct medical costs
Overall visit rates per patient-years were 9.05 for outpatient visits, 0.09 for inpatient hospitalizations, 0.73 for length of hospital stay, and 0.5 for psoriasis-related phototherapy. Bio-experienced patients had slightly lower rates of outpatient visits, and higher rates of inpatient hospitalization visits and length of stay than patients in the bio-naïve subgroup. As demonstrated by higher rate of visits per patient-year, higher overall disease burden was observed for bio-experienced patients. In turn, this translated into higher direct medical costs for bio-experienced patients (11,284,062 JPY per patient-year) compared to bio-naïve patients (9,364,392 JPY per patient-year). However, the visit rate per patient-year for phototherapy was greater for bio-naïve patients (0.76) compared to bio-experienced patients (0.17) ().
Table 4. Cross-sectional evaluation of HCRU and related direct medical costs in 2020.
Discussion
This is a real-world claims database study to evaluate biologic agents for the treatment of psoriasis in Japan during the timeframe of 2016 to 2020. We analyzed patient demographics and clinical characteristics, as well as comorbidities and use of concomitant medications in patients receiving biologic therapy for psoriasis. We also studied treatment patterns including switching and resumption of biologic therapies, in addition to treatment adherence, HCRU and direct medical costs for patients receiving biologic therapy. This study provides aggregated evidence for physicians to optimize selection of biologic therapy from the available treatment options and standardize disease management under a constrained HCRU setting in Japan.
The demographics of patients with psoriasis were similar across the treatment groups. The proportion of male patients (72.5%) treated with biologics for psoriasis was significantly higher than female patients (27.4%), which is consistent with a previous epidemiological survey (Citation31) and other studies in Japan (Citation23,Citation32). In addition, a higher mean CCI score was observed among patients treated with a TNFα inhibitor as their first-line biologic in our study, suggesting that patients receiving TNFα inhibitors were at higher risk of less favorable health prognosis. Consistent with this, a Danish cohort study showed that higher CCI score was associated with poor treatment response in patients with psoriatic arthritis treated with TNFα inhibitors (Citation33). The most frequent comorbid conditions related to recorded hospital visitations in the 12-month periods both prior to and after initiating biologic treatment were hypertension, cardiovascular diseases, hyperlipidemia, and diabetes in our study. Patients initiating IL-17 inhibitors used fewer concomitant medications, whereas in patients initiating TNFα inhibitors use of concomitant medications increased in general.
In other psoriatic arthritis and generalized pustular psoriasis claims database studies, similar to findings in our study, the most commonly reported comorbidities were hypertension, hyperlipidemia and type-2 diabetes, and the most frequently used concomitant medications were methotrexate and corticosteroids (Citation22,Citation34). However, in our study we observed decreased use of concomitant medications after initiating biologic treatment, except for TNFα inhibitors. Since the data were collected from a claims database, we could not investigate reasons for reduced use of concomitant medications; however, this likely reflects the overall favorable responses to biologic therapy.
With regards to biologic treatment patterns, TNFɑ inhibitors and IL-17 inhibitors were the most commonly utilized first-line biologic treatments. Advancement to second-line treatment mainly occurred within the same biologic class for first-line TNFɑ and IL-17 inhibitor users, and resumption of treatment for IL-12/23 inhibitor users. In contrast, only a small proportion of first-line IL-23 inhibitor users switched within the same class. In Japan, as access to approved biologic treatment is not an impediment, the practice of switching within the same mechanism of action class is probably due to physicians’ familiarity with given drug classes before switching to a relatively new one, and to ensure that treatment options are not completely exhausted for those patients who experience lack of response to the initial treatment (i.e. primary failure) (Citation7,Citation35,Citation36). Another potential reason for switching within the same class could be lack of availability of biologics in other newer classes.
Analysis of treatment switching, and resumption patterns indicated that bio-experienced patients had a shorter time to switch, and resumption compared to bio-naïve patients. This may reflect bio-experienced patients being somewhat more refractory to treatment, thus requiring switching sooner due to lack or loss of response. A European consensus report on treatment optimization of plaque psoriasis suggests that administration of treatment should be continuous and uninterrupted, given that approximately 20% of patients fail to regain PASI 75 response after resumption/reintroduction of same biologic therapy (Citation37). Considering better overall maintenance of response with continuous biologic therapy compared to intermittent therapy, frequent switching and resumption of the same biologic may lead to a high risk of disease recurrence.
In our study, overall adherence to biologic treatments was generally high; the most optimal adherence rates (nearest to 100% MPR), were observed for infliximab (TNFɑ inhibitor), ustekinumab (IL-12/23 inhibitor), and guselkumab (IL-23 inhibitor). Of note, on-site intravenous administration of infliximab could potentially enhance its adherence compared to other subcutaneous injections. High adherence may also be due to the National Health Insurance coverage available for all patients in Japan (Citation8). Similar to our findings, a previously published cohort study in Spain found that guselkumab demonstrated high adherence in psoriasis refractory to other biologic treatments (Citation38). Furthermore, in a US commercial database study both guselkumab and ustekinumab showed high adherence in psoriasis patients with comorbid psoriatic arthritis (Citation39). In our study, we found that TNFɑ inhibitors with highest adherence rates, were the first preferred treatment choice. Though, the evidence mentioned above demonstrated higher adherence rates for IL-23, the findings of our study suggest that IL-23 were the second most preferred choice of treatment. Patients with >100% MPR have refilled medication more frequently than per the recommended guideline.
In contrast, certolizumab pegol, adalimumab, and brodalumab did not meet the threshold for optimal adherence in our study. The observed low MPR for these biologics could be influenced by efficacy, safety, and dosing frequency, as these have shorter maintenance intervals compared to other biologics evaluated.
Other treatment preference studies also have shown that patients prefer less frequent injections of biologic agents (Citation10). In Japan, adalimumab, secukinumab, ixekizumab and brodalumab were approved and reimbursed for self-injection (Citation7,Citation8). Even though subcutaneous administration is generally convenient for patients, optimal adherence was not observed for these biologics. Notably, both ustekinumab (IL-12/23 inhibitor) and guselkumab (Il-23 inhibitor) per Japanese guidelines are not approved for self-injection in Japan and are administered in-clinic (Citation8); and direct treatment monitoring by the physicians may have contributed to the overall optimal adherence rates observed for these biologics in our study. In contrast to other biologics for psoriasis, a MPR >100% was seen for risankizumab in our study. This more than optimal adherence for risankizumab could suggest: i) a need for sooner than scheduled administration or shorter than labeled dosing frequency; or ii) physician preference for more frequent monitoring of patient response to a newer treatment option with a long dose interval. Risankizumab is administered subcutaneously in-clinic and has a relatively favorable dosing profile (maintenance doses administered once every 12 weeks) compared to other biologics. The less frequent dosing regimen may be preferred by patients resulting into better treatment adherence and this may be a potential reason for highest adherence observed for risankizumab in our study (Citation8,Citation40). In addition, in a global multicenter cohort study, high adherence rates were observed for risankizumab (Citation44).
In a self-reported medication adherence study, patients’ adherence to different types of treatments and factors affecting adherence demonstrated significantly better adherence for biologic therapies. Factors such as patients being too busy and patients being encumbered with troublesome application of topical treatments were associated with reduced adherence (Citation41). Treatment adherence is highly correlated with patient’s satisfaction with treatment and successful treatment management; hence it is important for physicians to evaluate patient preferences and satisfaction too (Citation42–44).
Though treatment adherence is an important factor to be considered for selecting an appropriate biologic treatment, factors that influence patient preferences for biologic treatments are also important considerations. Patient preferences for biologics are based on key attributes of effectiveness, low risk of adverse events, easy access to treatment, location of administration, and method of delivery of treatment according to findings from German and US-based registry studies (Citation10,Citation42,Citation43). In Japanese and Asia-Pacific regional survey studies, patient preferences for biologics were based on durable efficacy, low treatment burden (i.e. fewer injections/dosage frequency), low co‐payments, low risk of disease flares, and longer duration of maintaining clear skin (Citation44,Citation45). The findings of treatment adherence in our study, in addition to these observations, may help patients and physicians improve decision-making to optimize treatment satisfaction, which may indirectly influence better overall treatment outcomes.
For HCRU and overall medical costs, costs per patient-year were estimated to be higher for bio-experienced patients relative to bio-naïve patients. In contrast, utilization of phototherapy was low in the bio-experienced group compared to the bio-naïve group. This is in line with the Japanese psoriasis treatment guidelines, which recommend using other systemic therapies such as cyclosporin, etretinate, methotrexate, and apremilast, and phototherapy prior to using biologics (Citation7,Citation8). Furthermore, studies have shown that patients tend to not complete phototherapy cycles for the prescribed duration and terminate treatment early to avoid the risk of developing skin cancer with long term exposure, and switch to more effective treatments (Citation10,Citation46,Citation47).
Treatment switches may occur frequently due to many of the factors discussed above and may also affect HCRU and medical costs. A number of publications also suggest that frequent switching or discontinuation of biologics is associated with high medical costs and higher HCRU compared with maintaining treatment using the same biologic, further highlighting the clinical and economic effects of discontinuing or switching biologic therapies in patients with psoriasis (Citation48,Citation49).
Our results demonstrate that the bio-experienced group of patients had slightly higher rates of inpatient admission visits and more extended length of stay. Additionally, bio-experienced patients also demonstrated higher disease burden, as reflected by higher associated costs. This is in line with a recent retrospective US claims database analysis which showed that patients who switched treatment had higher total HCRU, driven by increased prescription and medical costs; also, inpatient costs were higher following the initiation of biologic therapy (Citation49,Citation50). In turn, selecting an optimal first-line treatment to improve adherence, achieve long-term treatment goals earlier, and reduce cycling through suboptimal treatments, may reduce overall HCRU and associated medical costs in the long-term.
There are several limitations in our study. This is a secondary health insurance registry study which includes anonymized claims data collected mainly for administrative purposes. Therefore, relevant variables for analyzing any potential benefits (such as improved tolerability, symptom severity, and health-related quality of life) influencing treatment lines and adherence are not available. There were potential sources of bias associated with JMDC population inclusion, such as partial representation of elderly patients (>65 years), as they may tend to drop out of the insurance system due to loss of employment related to advanced age or retirement, or overrepresentation of senior employees or dependent parents of employees covered under a family insurance plan. Further, some more recently reimbursed treatments (e.g. IL-23 inhibitors) were not fully captured due to limited data availability at the time of these analyses. Additionally, COVID-19 in the year 2020 might have impacted the findings related to treatment adherence. With accrual of more data over time, more robust analyses may be conducted in the future. In addition, the exact reasons for treatment discontinuation could not be captured, as such information is not available in the healthcare insurance claims data used for this study. Similarly, the use of claims data precludes assessment of whether patients with a claims record for receiving dispensed medications actually adhere to the treatment regimen as prescribed by the physician. Another limitation is the relatively small proportion of psoriasis patients receiving biologic therapy in the database; hence, our findings should be interpreted with caution and may not necessarily be representative of broader populations. Few biologics treated patients made it challenging to stratify the sample to analyze psoriatic arthritis and psoriasis separately, which could otherwise describe heterogeneity in the population. Lastly, we could not capture outpatient and inpatient costs related specifically to psoriasis since the JMDC reports total costs for all diagnoses per month. However, costs of psoriasis-related pharmaceuticals and procedures could be determined, and this study captured overall estimates of frequency of treatment switches and discontinuation, treatment patterns, HCRU, and costs of biologics for moderate to severely affected psoriasis population in Japan.
Conclusion
The findings in this study allow for further understanding of remaining unmet needs for Japanese psoriasis patients eligible for biologic treatment in a real-world setting that have not yet been explored in the existing literature. Among Japanese patients with psoriasis, most receiving IL-23 inhibitors previously received IL-17, TNFɑ or IL-12/23 inhibitors, denoting IL-23 inhibitors as the most commonly chosen option for biologic treatment switching. In general, optimal adherence patterns were observed for infliximab (administered by IV infusion), guselkumab, and ustekinumab; the lowest MPRs, reflecting the lowest adherence rates, were observed for patients who received certolizumab pegol, brodalumab and adalimumab, while the highest was observed for risankizumab. Overall, these findings potentially reflect the utilization of each respective biologic agent in the real-world setting. Higher disease burden was observed among bio-experienced compared to bio-naïve patients, based on higher overall medical costs reported here. Further research is required to better understand factors affecting the choice of treatment options, improve outcomes, and achieve adherence to chronic treatment. Nonetheless, this study provides valuable information on current treatment patterns, drug adherence, HCRU, and medical costs in a real-world setting for patients with psoriasis receiving biologic treatment in Japan. Results from this study may help in improving HCRU and cost management for psoriasis in the Japanese healthcare system over time.
Author contributions
Celine Miyazaki (Janssen Pharmaceutical K.K., Japan) developed the initial outline draft of this manuscript, including the data tables and figures from the results analysis of the study. All authors contributed to the development and review of this manuscript and confirm that they have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. All authors met ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the analyzed data, rigorously reviewed the data output and interpretation, and made the final decision about where to publish these data and approved submission to this journal.
Disclosures
Celine Miyazaki, Junya Masuda and Phiona I-Ching Tsai are employees of Janssen Pharmaceutical K.K., Japan at the time the study was completed and may own stock in Johnson & Johnson. Hidehisa Saeki received lecture fees from Mitsubishi Tanabe Pharma Corporation, Taiho Pharmaceutical Co., Ltd., Torii Pharmaceutical Co. Ltd., Maruho Co., Ltd., Kyowa Kirin Co., Ltd., AbbVie GK, Novartis Pharma K.K., Eli Lilly Japan K.K., LEO Pharma K.K., Celgene Corp., Janssen Pharmaceutical K.K., UCB Japan Co. Ltd. and/or research costs, Maruho Co., Ltd, AbbVie GK, LEO Pharma K.K; and/or scholarship donations from Taiho Pharmaceutical Co., Ltd., Maruho Co., Ltd., Eisai Co., Ltd., and Sun Pharma Japan Ltd. Mateo Delclaux Rodriguez-Rey is an employee of Paraxel International, London. Marta Natalia Stelmaszuk and Jonatan Freilich are employees of Parexel International, Sweden.
Supplemental Material
Download PDF (764.6 KB)Acknowledgement
The authors thank Sonali Satam, Ph.D, for providing writing support and Uma Kundu, MPharm, CMPPTM for additional editorial support (All SIRO Clinpharm Pvt. Ltd., Maharashtra, India).
Data availability
The data that support the findings of this study are available from JMDC Inc. Restrictions apply to the availability of these data, which were used under license for this study. Data could be available on request with the permission of JMDC Inc.
Additional information
Funding
References
- Goto H, Nakatani E, Yagi H, et al. Late-onset development of psoriasis in Japan: a population-based cohort study. JAAD Int. 2021;2:1–10. doi: 10.1016/j.jdin.2020.10.011.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi: 10.1001/2013.jamadermatol.406.
- Gui XY, Jin HZ, Wang ZJ, et al. Serum uric acid levels and hyperuricemia in patients with psoriasis: a hospital-based cross-sectional study. An Bras Dermatol. 2018;93(5):761–763. doi: 10.1590/abd1806-4841.20187547.
- Kamiya K, Kishimoto M, Sugai J, et al. Risk factors for the development of psoriasis. Int J Mol Sci. 2019;20(18):4347. doi: 10.3390/ijms20184347.
- Dhana A, Yen H, Yen H, et al. All-cause and cause-specific mortality in psoriasis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(5):1332–1343. doi: 10.1016/j.jaad.2018.12.037.
- Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med (Lausanne). 2021;8:743180. doi: 10.3389/fmed.2021.743180.
- Saeki H, Mabuchi T, Asahina A, et al. English version of Japanese guidance for use of biologics for psoriasis (the 2022 version). J Dermatol. 2022;50(5):e138–e150. doi: 10.1111/1346-8138.16691.
- Saeki H, Terui T, Morita A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47(3):201–222. doi: 10.1111/1346-8138.15196.
- Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18(11):2297. doi: 10.3390/ijms18112297.
- Tada Y, Ishii K, Kimura J, et al. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46(6):466–477. doi: 10.1111/1346-8138.14870.
- Sruamsiri R, Iwasaki K, Tang W, et al. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database. BMC Dermatol. 2018;18(1):5. doi: 10.1186/s12895-018-0074-0.
- Chastek B, White J, Van Voorhis D, et al. A retrospective cohort study comparing utilization and costs of biologic therapies and JAK inhibitor therapy across four common inflammatory indications in adult US managed care patients. Adv Ther. 2016;33(4):626–642. doi: 10.1007/s12325-016-0312-y.
- Gu T, Shah N, Deshpande G, et al. Comparing biologic cost per treated patient across indications among adult US managed care patients: a retrospective cohort study. Drugs Real World Outcomes. 2016;3(4):369–381. doi: 10.1007/s40801-016-0093-2.
- Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US medicare population. J Am Acad Dermatol. 2016;74(6):1057–1065.e4. doi: 10.1016/j.jaad.2016.01.048.
- Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016;30(7):1148–1158. doi: 10.1111/jdv.13611.
- Zweegers J, van den Reek JM, van de Kerkhof PC, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long-term drug-survival study from the BioCAPTURE registry. Br J Dermatol. 2016;175(2):340–347. doi: 10.1111/bjd.14552.
- Dávila-Seijo P, Dauden E, Carretero G, et al. Survival of classic and biological systemic drugs in psoriasis: results of the BIOBADADERM registry and critical analysis. J Eur Acad Dermatol Venereol. 2016;30(11):1942–1950. doi: 10.1111/jdv.13682.
- Pogácsás L, Borsi A, Takács P, et al. Long-term drug survival and predictor analysis of the whole psoriatic patient population on biological therapy in Hungary. J Dermatolog Treat. 2017;28(7):635–641. doi: 10.1080/09546634.2017.1329504.
- Iskandar IYK, Ashcroft DM, Warren RB, et al. Patterns of biologic therapy use in the management of psoriasis: cohort study from the british association of dermatologists biologic interventions register (BADBIR). Br J Dermatol. 2017;176(5):1297–1307. doi: 10.1111/bjd.15027.
- Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509–519. doi: 10.1111/bjd.16102.
- Masui S, Yonezawa A, Momo K, et al. Infliximab treatment persistence among Japanese patients with chronic inflammatory diseases: a retrospective Japanese claims data study. Biol Pharm Bull. 2022;45(3):323–332. doi: 10.1248/bpb.b21-00906.
- Inui K, Sato M, Esterberg E, et al. Treatment practices and costs among patients with psoriatic arthritis: a Japanese hospital claims database analysis. Mod Rheumatol. 2021;31(6):1179–1191. doi: 10.1080/14397595.2021.1886629.
- Umezawa Y, Nobeyama Y, Hayashi M, et al. Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol. 2013;40(12):1008–1013. doi: 10.1111/1346-8138.12353.
- Tada Y, Kim H, Spanopoulos D, et al. Treatment patterns, healthcare resource utilization, and costs in patients with moderate-to-severe psoriasis treated with systemic therapy in Japan: a retrospective claims database study. J Dermatol. 2022;49(11):1106–1117. doi: 10.1111/1346-8138.16543.
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100.
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44. doi: 10.2147/RMHP.S19801.
- JMDC Claims Database. Available from: https://www.jmdc.co.jp/en/jmdc-claims-database/.
- International Classification of Diseases. Tenth Revision (ICD-10): Centers for Disease Control and Prevention; [updated December 29, 2021. Available from: https://www.cdc.gov/nchs/icd/icd10.htm#:∼:text=International%20Classification%20of%20Diseases%2CTenth%20Revision%20(ICD%2D10)&text=The%20International%20Classification%20of%20Diseases,and%20presentation%20of%20mortality%20statistics.
- Ciaccio EJ. Use of artificial intelligence in scientific paper writing. Inf Med Unlocked. 2023;41:101253. doi: 10.1016/j.imu.2023.101253.
- Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310. doi: 10.1185/03007990903126833.
- Kamiya K, Oiso N, Kawada A, et al. Epidemiological survey of the psoriasis patients in the Japanese society for psoriasis research from 2013 to 2018. J Dermatol. 2021;48(6):864–875. doi: 10.1111/1346-8138.15803.
- Kishimoto M, Komine M, Kamiya K, et al. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J Dermatol. 2020;47(1):33–40. doi: 10.1111/1346-8138.15146.
- Ballegaard C, Højgaard P, Dreyer L, et al. Impact of comorbidities on tumor necrosis factor inhibitor therapy in psoriatic arthritis: a population-based cohort study. Arthritis Care Res (Hoboken). 2018;70(4):592–599. doi: 10.1002/acr.23333.
- Morita A, Kotowsky N, Gao R, et al. Patient characteristics and burden of disease in Japanese patients with generalized pustular psoriasis: results from the medical data vision claims database. J Dermatol. 2021;48(10):1463–1473. doi: 10.1111/1346-8138.16022.
- Yamamoto T, Ohtsuki M, Sano S, et al. Switching biologics in the treatment of psoriatic arthritis in Japan. J Dermatol. 2019;46(3):e113–e4. doi: 10.1111/1346-8138.14622.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44(9):1015–1019. doi: 10.1111/1346-8138.13860.
- Mrowietz U, de Jong EM, Kragballe K, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2014;28(4):438–453. doi: 10.1111/jdv.12118.
- Medina-Catalán D, Riera P, Pagès-Puigdemont N, et al. A cohort study of guselkumab in the treatment of psoriasis refractory to previous biologic therapies: effectiveness, safety and adherence. Int J Clin Pharm. 2022;44(3):725–730. doi: 10.1007/s11096-022-01400-z.
- Xu C, Teeple A, Wu B, et al. Treatment adherence and persistence of seven commonly prescribed biologics for moderate to severe psoriasis and psoriatic arthritis in a U.S. commercially insured population. J Dermatolog Treat. 2022;33(4):2270–2277. doi: 10.1080/09546634.2021.1950600.
- Blair HA. Risankizumab: a review in moderate to severe plaque psoriasis. Drugs. 2020;80(12):1235–1245. doi: 10.1007/s40265-020-01357-1.
- Chan SA, Hussain F, Lawson LG, et al. Factors affecting adherence to treatment of psoriasis: comparing biologic therapy to other modalities. J Dermatolog Treat. 2013;24(1):64–69. doi: 10.3109/09546634.2011.607425.
- Bolt T, Kobayashi H, Mahlich J. Patient and physician preferences for therapy characteristics for psoriasis: a discrete choice experiment in Japan. Pharmacoecon Open. 2019;3(2):255–264. doi: 10.1007/s41669-018-0104-1.
- Harrold LR, Stolshek BS, Rebello S, et al. Impact of prior biologic use on persistence of treatment in patients with psoriatic arthritis enrolled in the US corrona registry. Clin Rheumatol. 2017;36(4):895–901. doi: 10.1007/s10067-017-3593-x.
- Mahlich J, Sruamsiri R. Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence. 2016;10:1509–1519. doi: 10.2147/PPA.S110147.
- Tada Y, Jo SJ, Huang YH, et al. Uncovering the unmet needs among psoriasis patients in the Asia-Pacific region. J Dermatol. 2021;48(11):1665–1674. doi: 10.1111/1346-8138.16072.
- Farahnik B, Patel V, Beroukhim K, et al. Combining biologic and phototherapy treatments for psoriasis: safety, efficacy, and patient acceptability. Psoriasis (Auckl). 2016;6:105–111. doi: 10.2147/PTT.S98952.
- Thatiparthi A, Martin A, Liu J, et al. Risk of skin cancer with phototherapy in moderate-to-severe psoriasis: an updated systematic review. J Clin Aesthet Dermatol. 2022;15(6):68–75.
- Schmitt-Egenolf M, Freilich J, Stelmaszuk-Zadykowicz NM, et al. Drug persistence of biologic treatments in psoriasis: a swedish national population study. Dermatol Ther (Heidelb). 2021;11(6):2107–2121. doi: 10.1007/s13555-021-00616-7.
- Feldman SR, Tian H, Wang X, et al. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. Claims database. J Manag Care Spec Pharm. 2018;25(4):479–488. doi: 10.18553/jmcp.2018.18308.
- Hur P, Kim N, Dai D, et al. Healthcare cost and utilization associated with biologic treatment patterns among patients with psoriatic arthritis: analyses from a large US claims database. Drugs Real World Outcomes. 2021;8(1):29–38. doi: 10.1007/s40801-020-00217-4.