Burden of atopic dermatitis
Atopic dermatitis (AD) is an intensely pruritic, chronic, relapsing, inflammatory skin disease that commonly presents in early childhood, but also affects adults, and severely impacts the quality of life of patients and their families (Citation1). The incidence of AD has increased globally and it is one of the most common chronic inflammatory skin diseases in the world, with a US prevalence of 10–20% for children and adolescents and approximately 7% for adults (Citation2–6). In a global burden of disease study, AD was ranked highest among skin diseases and in the top 15 of all non-fatal diseases (Citation7). The economic burden of AD is also considerable, with average annual costs estimated to be more than $5.3 billion in the US in 2015 (Citation8), and annual costs per patient estimated at more than $10,000 in the US (in 2018) (Citation9) and €15,000 in Europe (in 2019) (Citation10).
Most patients with AD are diagnosed in early childhood, but the disease can begin at any age (Citation11). The cardinal and most burdensome symptom of AD is intense pruritus, which has a profound negative influence on sleep, physical and mental development, and the overall quality of life of patients, their families, and caregivers (Citation2,Citation12–14). Sleep impairments in children with AD are associated with increased risk of short stature, metabolic syndrome, mental illness, and neurocognitive dysfunction.
The individual pattern of disease in AD is variable, with acute exacerbations that can affect different areas of the body, including sensitive and intertriginous areas that can be difficult to treat. Symptoms of AD are also influenced by dynamic environmental factors such as temperature, humidity, allergens, irritants, and pollutants (Citation15). Consequently, AD symptoms can be persistent or cycle over time so that a patient can have mild, moderate, or severe symptoms at different times, requiring frequent treatment adjustments to several topical and/or systemic therapies to manage their disease (Citation16).
There is increased recognition that AD presents a broad spectrum of clinical phenotypes, reflective of its underlying pathophysiologic heterogeneity (Citation17–19). Evidence for the clinical relevance of AD endotype variability is also apparent in the widely differing outcomes observed with current therapies and based on a range of factors, such as age, skin type, ethnicity, and disease severity (Citation19). Given the heterogeneity in the natural course of AD, and the unpredictable individual trajectory of the disease, it is perhaps unsurprising that there are significant gaps in available therapies and that no single intervention has proven effective for all patients across the spectrum of disease severity or throughout the disease course.
In addition to treatments that can address the variable signs, symptoms, and needs of patients with AD, there is a gap in the armamentarium for therapeutics that can alter the natural course of disease (Citation20). Disease-modifying therapy for AD could achieve lasting remission and/or prevent progression of the atopic march, because AD increases the risk for the development of other allergic diseases, which is particularly important in children where disease modification may prevent the development of allergies and asthma (Citation21).
Gaps in current treatment
There is no curative therapy for AD and conventional management has relied on corticosteroids (topical and systemic), phototherapy, topical calcineurin inhibitors, topical phosphodiesterase 4 (PDE4) inhibitors, and systemic immunosuppressants such as cyclosporine. Topical corticosteroids are the mainstay of treatment for most patients with AD, regardless of disease severity; however, current options have well-documented limitations and safety concerns, including restrictions on duration, extent, and location of application (Citation22). Furthermore, symptoms can rapidly recur after discontinuation of corticosteroids.
Since 2000, the US Food and Drug Administration has approved four systemic (Citation23–26) and four topical AD medications (Citation27–30) (). Of the systemic medications, none are approved for mild AD, and all either require injection and/or carry warnings and age restrictions due to safety concerns. Of the topical medications, only one is approved for the treatment of severe AD (tacrolimus), with its use restricted due to safety concerns, to second-line, short-term, non-continuous therapy in non-immunocompromised patients who fail to respond to other topical prescription treatments for AD. The topical AD medications are associated with adverse events of application site pain/burning/stinging (crisaborole, tacrolimus, and pimecrolimus), have restrictions on duration of use (tacrolimus and pimecrolimus), or have warnings and restrictions based on age and maximum body surface area treated (ruxolitinib). Current topical medications also require twice-daily application, which is known to be burdensome and detrimental to patient/caregiver satisfaction and adherence to therapy. Furthermore, as with corticosteroids, signs and symptoms of AD often recur soon after discontinuation of current topical medications.
Table 1. Tapinarof cream 1% compared with treatments for atopic dermatitis approved by the US FDA since 2000: Regulatory restrictions, warnings, and common adverse events.
To address these gaps, topical medications that meet the diverse needs of patients with AD across the full spectrum of disease severity with mechanisms of action that might significantly alter the course of the disease would be useful. Ideally, improved topical medications would achieve complete disease clearance with once-daily application, rapid control of symptoms such as itch, favorable safety and tolerability, and minimal systemic absorption with no drug-drug interactions; such mediations would be suitable for pediatric patients and for application to sensitive skin areas; and would have a cosmetically elegant formulation. Additional characteristics of improved topical mediations for AD include durable disease control off therapy, an absence of tachyphylaxis on therapy, and restoration of a healthy skin barrier. An ultimate goal is for new topical medications with efficacy that is maintained after treatment discontinuation, allowing treatment to be paused and restarted as needed, with the potential for disease modification to prevent disease progression and the atopic march.
The aryl hydrocarbon receptor
Advances in our understanding of mechanisms that underlie the pathogenesis and phenotypes of AD offer potential new targets for pharmacologic intervention. The aryl hydrocarbon receptor (AhR) is a ligand-dependent transcription factor that acts as a master regulator and communication node for multiple signaling pathways involved in skin homeostasis, immune responses, and epithelial barrier function (Citation36,Citation37). Targeting a transcription factor such as AhR represents a novel approach to therapy, with the potential to address several gaps in the current treatment armamentarium for AD.
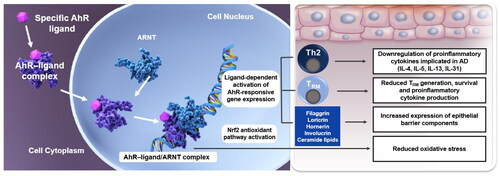
As an aryl hydrocarbon receptor agonist, tapinarof, a nonsteroidal topical medication, has a profile that fundamentally differs from available AD treatments. Tapinarof specifically binds to, and activates, AhR to downregulate proinflammatory cytokines, reduce oxidative stress, and upregulate skin barrier protein expression () (Citation38,Citation39,Citation43). Tapinarof also inhibits the generation, persistence, and cytokine production of resident memory T cells in the skin (Citation42), which may explain the remittive effect demonstrated in patients with psoriasis who maintained clear or almost clear skin for approximately 4 months after cessation of treatment in a long-term phase 3 trial (Citation45). In the context of AD, a mechanism of action with potential disease-modifying effects may be especially important for children, where restoration of the skin barrier could impact allergic sensitization and the atopic march and offer the opportunity for treatment-free periods.
Figure 1. Ligand-specific binding and activation of the aryl hydrocarbon receptor in the treatment of atopic dermatitis (Citation36,Citation38–44). Ligand-specific binding and activation of the aryl hydrocarbon receptor downregulates proinflammatory cytokines, reduces oxidative stress, upregulates expression of skin barrier components and inhibits the generation, persistence, and cytokine production of resident memory T cells in the skin. Some of the proposed downstream effects of tapinarof in AD are distinct from its mechanism of action in psoriasis. The proposed mechanism of action of tapinarof in psoriasis is described by Bissonnette et al. (Citation36). AhR: aryl hydrocarbon receptor; ARNT: aryl hydrocarbon receptor nuclear translocator; DNA: deoxyribonucleic acid. Th2: T helper 2 cell; TRM: resident memory T cell.
Preliminary results from the ADORING trials (NCT05014568; NCT05032859), presented as late breaking news at the European Academy of Dermatology and Venereology 2023 in Berlin (Citation34,Citation35), indicate that tapinarof cream 1% once daily has a rapid onset of action with robust efficacy and tolerability for both investigator- and patient-assessed outcomes in patients with AD down to 2 years of age. The primary efficacy endpoint of a Validated Investigator Global Assessment for Atopic Dermatitis™ response at Week 8, defined as a score of 0 or 1 and at least a 2-grade improvement from baseline, was achieved with high statistical significance in the tapinarof groups versus vehicle in both trials (approximately 45% versus 14% and 46% versus 18% in each trial, respectively, both p < 0.0001). The trials were vehicle controlled, but comparing outcomes across trials of AD therapies suggests that tapinarof cream is at least similar, if not superior, in efficacy compared with the other topical AD therapies, and even the injectable drugs.
The long-term extension trial, ADORING 3 (ongoing in 2023–24), will confirm if the durability (lack of tachyphylaxis), and potential to achieve complete clearance with disease control maintained while off therapy (i.e., a ‘remittive’ effect), that was observed with tapinarof cream 1% once daily in the phase 3 PSOARING psoriasis long-term extension trial (Citation45), will be replicated in patients with AD. ADORING 3 will also provide further evidence regarding potential long-term use of tapinarof cream 1% once daily in patients down to 2 years of age with AD of any severity, including mild, to achieve consistent efficacy when used continuously or intermittently as needed, based on individual patient responses.
Targeting AhR to modulate multiple upstream actions provides the potential to address the diverse endotypes of AD, and the dynamic and variable individual course of disease, including potential disease-modifying effects (Citation10,Citation20). A remittive effect off therapy, as demonstrated in the treatment of plaque psoriasis with the AhR agonist tapinarof (Citation45), could offer the opportunity for treatment that can be stopped and restarted, as needed, without concerns regarding rebound phenomena. Beyond the above-mentioned attributes, tapinarof has demonstrated no drug-drug interactions and minimal-to-no systemic absorption (Citation33,Citation46). The targeted effect of tapinarof in the skin and minimal systemic exposure confer an intrinsically low potential for drug-drug interactions and systemic side effects. These properties have the potential to improve the treatment of AD by rapidly relieving burdensome symptoms including pruritus, improving quality of life, and reducing reliance on systemic agents.
Discussion and conclusions
AD is a complex disease that encompasses a spectrum of clinical presentations reflective of underlying biologic heterogeneity, resulting in variable outcomes with current therapeutic options. Despite recent developments, key gaps remain in the AD therapeutic armamentarium for topical medications that can provide robust and long-term efficacy, across the spectrum of severity, without restrictions on the duration, location, or extent of application for all patients with AD, regardless of age and disease characteristics. Preliminary results from the ADORING trials indicate that targeting AhR is a valid approach that may address these gaps, with the potential to significantly transform the treatment of AD. Once-daily tapinarof is not associated with application site burning or safety concerns that often restrict the use of current options.
The role of AhR in regulating epidermal barrier function and skin homeostasis underlies the potential of this strategy to address diverse individual patient needs in AD. Long-term data are needed to demonstrate whether this strategy has the potential for disease modification in AD, including reducing the risk of developing atopic comorbidities, such as allergies and asthma.
In view of the clinical effectiveness of tapinarof cream demonstrated in inflammatory skin diseases, targeting AhR may offer a novel therapeutic strategy in other diseases, beyond allergies and asthma, involving homeostasis and immune function of epithelial barrier tissues. New formulations targeting specific tissues may enable the development of targeted AhR therapies for inflammatory gastrointestinal, ophthalmic, and neurological diseases.
Disclosure statement
L.F.E. has served as a consultant, advisor, or investigator for AbbVie, Amgen, Arcutis, Aslan, Dermavant Sciences, Inc., Eli Lilly, Forté, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, and UCB Pharma. J.I.S. has received honoraria or grants, and/or has served as a consultant, an advisory board member, or speaker for Afyx, AOBiome, Arena, Asana, BiomX, Bluefin Biomedicine, Bodewell, Boehringer Ingelheim, Celgene, Dermavant Sciences Inc., Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Kiniksa, LEO Pharma, Luna, Menlo, Novartis, Pfizer, RAPT, Regeneron, and Sanofi Genzyme. A.A.H. has received research support paid to the medical school from AbbVie, Arcutis, Dermavant Sciences Inc., and Pfizer; honoraria received from GSK, Sanofi Regeneron, and Ortho Dermatologics (as part of a Data Safety Monitoring Board); honoraria received from Dermavant Sciences, Inc., Incyte, LEO Pharma, Pfizer, Arcutis, Sun Pharma, Galderma, Novan, and Verrica. M.B. has been an investigator for Regeneron, Sanofi, and Incyte, and served as a consultant and/or an advisory board member for AbbVie, Amgen, Dermavant Sciences, Inc., Eli Lilly, Incyte, Janssen, LEO Pharma, Pfizer, Regeneron, and Sanofi Genzyme.
Additional information
Funding
References
- Eichenfield LF, Stein Gold LF. Practical strategies for the diagnosis and assessment of atopic dermatitis. Semin Cutan Med Surg. 2017;36(2 Suppl 2):1–5. doi:10.12788/j.sder.2017.009.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta- analysis. Int J Dermatology. 2020;59(4):e75–e91. doi:10.1111/ijd.14763.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in america study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi:10.1016/j.jid.2018.08.028.
- Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43(4):649–655. doi:10.1067/mjd.2000.107773.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 national survey of children’s health. J Invest Dermatol. 2011;131(1):67–73. doi:10.1038/jid.2010.251.
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25(3):107–114. doi:10.1097/DER.0000000000000034.
- Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017. Br J Dermatol. 2021;184(2):304–309. doi:10.1111/bjd.19580.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the national eczema association. J Invest Dermatol. 2017;137(1):26–30. doi:10.1016/j.jid.2016.07.012.
- Wang X, Boytsov NN, Gorritz M, et al. US health care utilization and costs in adult patients with atopic dermatitis by disease severity. J Manag Care Spec Pharm. 2022;28(1):69–77. doi:10.18553/jmcp.2022.28.1.69.
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21(1):21–40. doi:10.1038/s41573-021-00266-6.
- Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management. Am J Clin Dermatol. 2019;20(6):771–779. doi:10.1007/s40257-019-00453-7.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis. Impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi:10.1111/j.1525-1470.2005.22303.x.
- LeBovidge JS, Kelley SD, Lauretti A, et al. Integrating medical and psychological health care for children with atopic dermatitis. J Pediatr Psychol. 2007;32(5):617–625. doi:10.1093/jpepsy/jsl045.
- Meltzer LJ, Flewelling KD, Jump S, et al. Impact of atopic dermatitis treatment on child and parent sleep, daytime functioning, and quality of life. Ann Allergy Asthma Immunol. 2020;124(4):385–392. doi:10.1016/j.anai.2019.12.024.
- Pan Z, Dai Y, Akar-Ghibril N, et al. Impact of air pollution on atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol. 2023;65(2):121–135. doi:10.1007/s12016-022-08957-7.
- Abuabara K, Margolis DJ, Langan SM. The long-term course of atopic dermatitis. Dermatol Clin. 2017;35(3):291–297. doi:10.1016/j.det.2017.02.003.
- Suárez-Fariñas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132(2):361–370. doi:10.1016/j.jaci.2013.04.046.
- Thijs JL, de Bruin-Weller MS, Hijnen D. Current and future biomarkers in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37(1):51–61. doi:10.1016/j.iac.2016.08.008.
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta- analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80(2):390–401. doi:10.1016/j.jaad.2018.09.035.
- Bieber T. Disease modification in inflammatory skin disorders: opportunities and challenges. Nat Rev Drug Discov. 2023;22(8):662–680. doi:10.1038/s41573-023-00735-0.
- Davidson WF, Leung DY, Beck LA, et al. Report from the national institute of allergy and infectious diseases workshop on "atopic dermatitis and the atopic march: mechanisms and interventions. J Allergy Clin Immunol. 2019;143(3):894–913. doi:10.1016/j.jaci.2019.01.003.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83–93.
- FDA Tralokinumab-ldrm (ADBRY™) Prescribing Information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761180s001lbl.pdf
- FDA. Dupilumab (DUPIXENT®) Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761055s042lbl.pdf
- FDA. Abrocitinib (CIBINQO™). Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- FDA. Upadacitinib (RINVOQ®) Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- FDA. Crisaborole (EUCRISA™) Prescribing Information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/207695s007s009s010lbl.pdf
- FDA. Ruxolitinib (OPZELURA™) Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- FDA. Pimecrolimus (ELIDEL®) Prescribing Information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021302s018lbl.pdf
- FDA. Tacrolimus (PROTOPIC®) Prescribing Information. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050777s018lbl.pdf
- Ruer-Mulard M, Aberer W, Gunstone A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26(5):551–558. doi:10.1111/j.1525-1470.2009.00981.x.
- Eichenfield LF, Gower RG, Xu J, et al. Once-daily crisaborole ointment, 2%, as a long-term maintenance treatment in patients aged ≥ 3 months with mild-to-moderate atopic dermatitis: a 52-week clinical study. Am J Clin Dermatol. 2023;24(4):623–635. doi:10.1007/s40257-023-00780-w.
- FDA. Tapinarof (VTAMA®) cream Prescribing Information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Silverberg JI, Eichenfield LF, Hebert AA, et al. Tapinarof cream 1% once daily: significant efficacy in the treatment of moderate to severe atopic dermatitis in two pivotal phase 3 trials in adults and children down to 2 years of age. In: Late breaking news oral presentation at the European academy of dermatology and venereology, Berlin, Germany, 2023.
- Simpson EL, Silverberg JI, Bissonnette R, et al. Rapid and early onset of itch relief with tapinarof cream 1% once daily in two pivotal phase 3 trials in adults and children down to two years of age with moderate to severe atopic dermatitis. In: Late breaking news oral presentation at the european academy of dermatology and venereology., Berlin, Germany, 2023.
- Bissonnette R, Gold LS, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor–modulating agent. J Am Acad Dermatol. 2021;84(4):1059–1067. doi:10.1016/j.jaad.2020.10.085.
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40(6):989–1001. doi:10.1016/j.immuni.2014.04.019.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67(2):259–279. doi:10.1124/pr.114.009001.
- Furue M, Hashimoto-Hachiya A, Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2019;20(21):5424. doi:10.3390/ijms20215424.
- Furue M, Tsuji G, Mitoma C, et al. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J Dermatol Sci. 2015;80(2):83–88. doi:10.1016/j.jdermsci.2015.07.011.
- Furue M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: pathogenic implications in atopic dermatitis. Int J Mol Sci. 2020;21(15):5382. doi:10.3390/ijms21155382.
- Mooney N, Teague J, Gehad A, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. J of Skin. 2023;7(2):s194. doi:10.25251/skin.7.supp.194.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137(10):2110–2119. doi:10.1016/j.jid.2017.05.004.
- Sutter CH, Azim S, Wang A, et al. Ligand activation of the aryl hydrocarbon receptor upregulates epidermal uridine diphosphate glucose ceramide glucosyltransferase and glucosylceramides. J Invest Dermatol. 2023;143(10):1964–1972.e4. doi:10.1016/j.jid.2023.03.1662.
- Strober B, Gold LS, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87(4):800–806. doi:10.1016/j.jaad.2022.06.1171.
- Jett JE, McLaughlin M, Lee MS, et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics. Am J Clin Dermatol. 2022;23(1):83–91. doi:10.1007/s40257-021-00641-4.

