Dear Editor,
Apremilast, a phosphodiesterase 4 inhibitor, is commonly prescribed for the treatment of psoriasis [Citation1]. Recent studies indicate its potential efficacy in managing pustular pustulosis of the palms and toes (PPP) [Citation2], the primary manifestation of SAPHO syndrome. Prior literature has also proposed Apremilast as a potential therapeutic option for SAPHO syndrome [Citation3]. However, we present a case where Apremilast demonstrated ineffectiveness as an initial treatment for SAPHO syndrome.
Case report
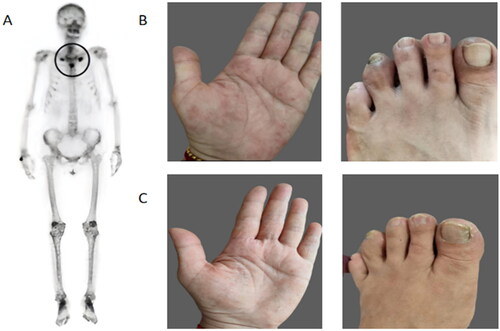
A 53-year-old middle-aged woman presented to a local hospital with pain in the anterior chest wall, clavicle, sternum, and scapula, as well as palmar pustulosis pilaris (PPP) for 1 year, and the results of a whole-body bone imaging showed (): increased radioactivity uptake bilaterally in the first thoracic ribcage joints, sternal stalks, and sternal body junctions, with the typical bull’s-eye shadow. Based on the patient’s clinical presentation and imaging, she was diagnosed with SAPHO syndrome. NSAIDs drugs (etoricoxib 60 mg qd) and apremilast (30 mg bid) were given, and after 3 months, the rash as well as the bone pain symptoms did not improve and a waxing toe developed (). After 2 months of treatment with tofacitibine (5 mg tid), the patient’s PPP and waxed toe improved significantly (), but she still had bone pain. After one cycle of treatment with bisphosphonates (zoledronic acid 4 mg in a single injection for 3 consecutive days), the patient’s bone pain and PPP symptoms gradually improved.
Figure 1. (A) Technetium99m-methyl diphosphonate whole-body bone scintigraphy showed abnormal accumulation of radioactivity in the bilateral sternoclavicular joints. (B) Prior to tofacitib treatment, the patient developed skin lesions and waxy toes. (C) After tofacitib treatment, the patient’s skin lesions and waxy toe symptoms improved significantly.
Discussion
SAPHO syndrome is a rare disease caused by autoimmune dysfunction manifested by osteoarthritis and skin disease.Characteristic osteoarthritis includes osteitis, osteomalacia, synovitis and arthropathy, and skin lesions include metacarpophalangeal pustulosis and severe acne [Citation4]. The pathogenesis of SAPHO syndrome is not clear.Tumor Necrosis Factor (TNF-a),Interleukin (IL)-1, 17, 23 are involved in inflammatory pathway expression, and inflammation activates Janus kinase (JAK), which initiates intracellular message transcription. Bone erosion due to pro-inflammatory cytokine expression, osteoclast formation and activation leads to cartilage destruction [Citation5]. Therefore SAPHO syndrome is mainly treated with anti-inflammatory and antiosteolysis therapy. Previous literature has demonstrated the effectiveness of Apremilast treatment and this may be after anti-inflammatory and bisphosphonate therapy. Apremilast, as a phosphodiesterase 4 inhibitor, prevents intracellular hydrolysis of cyclic adenosine monophosphate (cAMP), which results in the inhibition of a number of downstream pathways of cytokines, as well as the activation of protein kinase A and the increase of IL-10 [Citation2]. Since apremilast has weaker anti-inflammatory effects than traditional antirheumatic drugs and does not have anti-osteolytic capacity, we switched to tofacitib and bisphosphonates. Tofacitib, which is classified as a JAK inhibitor (JAKi), is a novel anti-inflammatory drug that exhibits more potent anti-inflammatory activity than conventional drugs and is able to block a number of cytokines including IL-1, IL-17, IL-23, and TNF-α. In our editorial on the successful treatment of SAPHO syndrome with tofacitib, baricitinib, and upatinib in the JAKi family, JAKi was effective in not only improving bone pain, skin damage, and reducing inflammation levels to near-normal levels, but also spinal cord edema, and waxy toes in patients with SAPHO [Citation6,Citation7]. JAKi shows potential for the treatment of SAPHO syndrome. The main effects of bisphosphonates are anti-bone resorption and bone reconstruction, as well as anti-inflammatory and immunomodulatory effects. During inflammation, bisphosphonates inhibit macrophage proliferation and chemotaxis and induce apoptosis. Inhibition of the release of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 not only prevents bone loss and fracture development, but also prevents structural joint damage by reducing paraprosthetic bone erosion [8]. Thus, bisphosphonates ameliorate osteitis in SAPHO syndrome through a dual mechanism of anti-osteoclast and anti-inflammatory. The efficacy of pamphiphosphate (1 mg/kg/day, 3 days and 3 months after baseline) in patients with SAPHO syndrome was evaluated in our previous study. After 12 months of follow-up, significant reductions in erythrocyte sedimentation rate, high-sensitivity C-reactive protein levels, visual analog scores, osteocalcin, and bone marrow edema (BME) were observed compared to baseline [Citation9]. PPP remission rates were >50% [Citation10]. This study demonstrated the ability of pamphiphosphate to provide efficient and rapid symptomatic relief in SAPHO syndrome, effective improvement of skin symptoms, and long-term remission effects on inflammation and spinal BME. Wang et al. reported successful treatment of osteoarthritis in SAPHO syndrome using zoledronic acid monotherapy, with patients experiencing relief of bone pain symptoms after the first course of treatment and complete resolution of bone pain symptoms after the fourth course [Citation11]. In addition, C.S et al. followed up several patients treated with zoledronic acid. After systemic treatment, the patients experienced a gradual reduction in clinical symptoms until they reached complete remission, magnetic resonance imaging (MRI) showed reduced or no inflammation at the lesion, and bone scans showed a reduction in tracer uptake compared to the previous period or reached complete normalization [Citation12]. In our team’s treatment with zoledronic acid, we have found that zoledronic acid has similar therapeutic effects to pamidophosphate with fewer side effects [Citation13].
In conclusion, in our case report, Apremilast could not be used as an initial treatment for SAPHO syndrome, JAKi and bisphosphonates had better therapeutic effects, the therapeutic response of SAPHO syndrome to different anti-inflammatory drugs may reflect its complex pro-inflammatory pathways, and control of inflammation and anti-bone metabolism remain the mainstay of treatment for SAPHO syndrome.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:1–3. doi:10.2147/PTT.S303634.
- Spencer RK, Elhage KG, Jin JQ, et al. Apremilast in palmoplantar psoriasis and palmoplantar pustulosis: a systematic review and meta-analysis. Dermatol Ther (Heidelb). 2023;13(2):437–451. doi:10.1007/s13555-022-00877-w.
- Adamo S, Nilsson J, Krebs A, et al. Successful treatment of SAPHO syndrome with apremilast. Br J Dermatol. 2018;179(4):959–962. doi:10.1111/bjd.16071.
- Li C, Wang H, Jiang H, et al. Family aggregation and prevalence of other autoimmune diseases in SAPHO syndrome. Heliyon. 2023;9:e21541. doi:10.1016/j.heliyon.2023.e21541
- Cheng W, Li F, Tian J, et al. New insights in the treatment of SAPHO syndrome and medication recommendations. J Inflamm Res. 2022;15:2365–2380. doi:10.2147/JIR.S353539.
- Yue Q, Ma M, Liu S, et al. JAKi: can it be used to treat SAPHO syndrome? Int J Rheum Dis. 2023. doi:10.1111/1756-185X.14930.
- Jiang H, Lv L, Lin Z, et al. SAPHO syndrome can cause sausage finger lesions. RMD Open. 2023;9(3):e003335. doi:10.1136/rmdopen-2023-003335.
- Peris P, Monegal A, Guañabens N. Bisphosphonates in inflammatory rheumatic diseases. Bone. 2021;146:115887. doi:10.1016/j.bone.2021.115887.
- Li C, Zhao Y, Zuo Y, et al. Efficacy of bisphosphonates in patients with synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: a prospective open study. Clin Exp Rheumatol. 2019;37(4):663–669.
- Wu N, Zhao Y, Tao W, et al. A single cohort, open-label study of the efficacy of pamidronate for palmoplantar pustulosis in synovitis, acne, pustulosis, hyperostosis and osteitis (SAPHO) syndrome. Clin Exp Rheumatol. 2020;38(6):1263–1264.
- Wang CR, Tsai YS, Whang-Peng J. Zoledronic acid monotherapy improves osteoarticular involvement in SAPHO syndrome. Scand J Rheumatol. 2020;49(5):419–421. doi:10.1080/03009742.2020.1769179.
- Rodriguez-García SC, Castellanos-Moreira R, Muxí A, et al. Imaging follow-up of SAPHO syndrome treated with zoledronic acid. J Clin Rheumatol. 2020;26(6):e155–e157. doi:10.1097/RHU.0000000000001025.
- Liu S, Yin D, Lin Z, et al. Short-term efficacy of zoledronic acid in the treatment of 30 cases of SAPHO syndrome. Clin Exp Rheumatol. 2023. doi:10.55563/clinexprheumatol/zpgyz9.

