Abstract
Introduction
Isotretinoin is a widely used, effective medication for moderate to severe acne. It is typically used for several months, which necessitates regular laboratory monitoring. However, consensus on the optimal assessment frequency is lacking.
Method
This is a single-center retrospective study on 1182 patients who received isotretinoin for acne at the Dermatology Clinic in Jordan University Hospital over 5 years.
Results
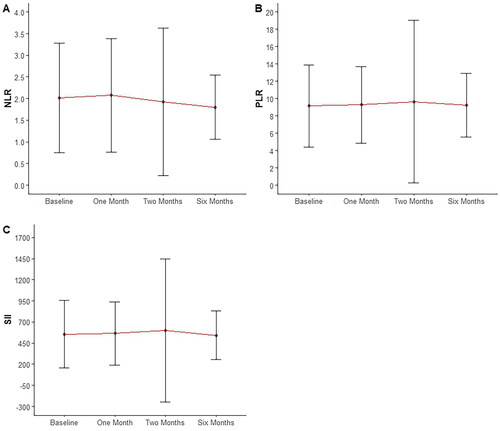
Of the 1182 patients, 892 (76.57% females) met the inclusion criteria. An increase in the proportion of patients with abnormal triglycerides and total cholesterol levels from baseline to the sixth month was observed (p < 0.05). Conversely, differences in the number of patients with abnormal AST, ALT, and CBC were not found throughout treatment (p > 0.05). Moreover, there was a decrease in the neutrophil-to-lymphocyte ratio (NLR) ratio and systemic inflammatory index (SII) after the sixth month of isotretinoin treatment compared to the baseline (p = 0.012 and p = 0.021, respectively).
Conclusions
We found that a baseline cholesterol level of 163.9 mg/dl and a baseline triglycerides level of 85.5 mg/dL are highly specific and sensitive in detecting grade 1 abnormalities at the one-month follow-up. This novel prediction approach serves as an effective risk stratification method for isotretinoin acne patients.
Introduction
Acne vulgaris is highly prevalent, impacting approximately 80% of young individuals worldwide. It is a chronic inflammatory condition originating in the pilosebaceous units (Citation1–4).
Oral isotretinoin was approved in 1982 by the US Food and Drug Administration to treat acne vulgaris (Citation5). It reduces sebaceous gland activity and size, normalizes sebaceous follicle keratinization, and decreases the Propionibacterium acnes count (Citation2,Citation6). Additionally, it plays a crucial anti-inflammatory role in acne treatment (Citation7). Previous studies have explored isotretinoin’s impact on inflammatory markers related to complete blood count (CBC), such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) (Citation8–10). The systemic immune-inflammatory index (SII), calculated as neutrophil x platelet/lymphocyte, has also been proposed as a more useful indicator of isotretinoin’s anti-inflammatory effect (Citation8,Citation11).
Despite its efficacy, isotretinoin may cause laboratory abnormalities, including hyperlipidemia and transaminitis, necessitating regular monitoring (Citation2–4). This study aims to explore the isotretinoin impact on alanine transaminase (ALT), aspartate aminotransferase (AST), lipid panel, CBC, and inflammatory parameters (NLR, PLR, and SII) in Jordanian acne patients, investigating the timing and severity of these changes.
Methodology
We conducted a descriptive retrospective study at Jordan University Hospital, a leading academic center in Jordan, involving patients receiving oral isotretinoin for acne vulgaris from 2018 to 2023. Patients underwent CBC, transaminases, and lipid panel testing at least twice: at baseline and during follow-up visits. Demographic, clinical, and laboratory data were collected from 892 eligible patients out of 1182 reviewed files. Descriptive analysis used IBM SPSS version 23 (IBM Corp., Armonk, NY, USA), presenting means and standard deviations for continuous variables as well as frequencies and percentages of categorical data. Lab results at baseline, follow-up, and end therapy were compared, assessing trends, monthly check percentages, and the impact of baseline cholesterol and triglycerides on follow-up abnormalities. The changes in inflammatory markers during treatment were also studied. We graded lab results using the Common Terminology Criteria for Adverse Events (CTCAE) version 5. A P-value of less than 0.05 was defined as statistical significance. We adhered to the Declaration of Helsinki’s guiding principles. We treated with the utmost confidentiality and privacy all of the collected data, and the institutional review board gave their approval to the study protocol.
Results
Demographics and lab monitoring trends in isotretinoin-treated acne patients
Our study of 892 individuals treated with isotretinoin for acne (76.57% females) revealed an average age and standard deviation of 21.83 ± 6.48 and an interquartile range of 5. At baseline, the majority had triglycerides (93.27%), total cholesterol (93.05%), AST (95.63%), and ALT (95.29%) assessed. By the sixth month, around 45% of patients had these monitored. White blood cell (WBC) and platelet count assessments decreased to 5.61% and 5.49%, respectively ().
Table 1. Percentage of patients on isotretinoin with labs checked by month.
Changes in lab results during isotretinoin therapy in acne patients
At baseline, 94.23%, 89.43%, and 89.04% of patients had normal triglycerides, hemoglobin, and total cholesterol, respectively. An increase in abnormal triglycerides and total cholesterol levels from baseline to the sixth month was observed (p-value < 0.001). For triglycerides, grade 1 abnormalities increased from 5.53% to 19.80% by the sixth month. A concomitant increment in LDL and a decrease in HDL were observed in the first and second months, but not in the sixth. In contrast, differences in the number of patients with abnormal AST and ALT levels were not found throughout treatment (p-value > 0.05) ().
Table 2. Number of patients with a lab abnormality by month of therapy.
The Influence of baseline cholesterol levels on follow-up abnormalities
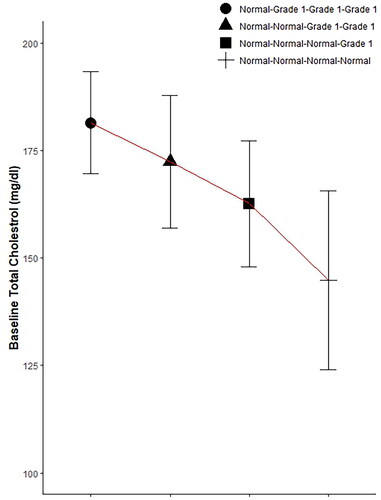
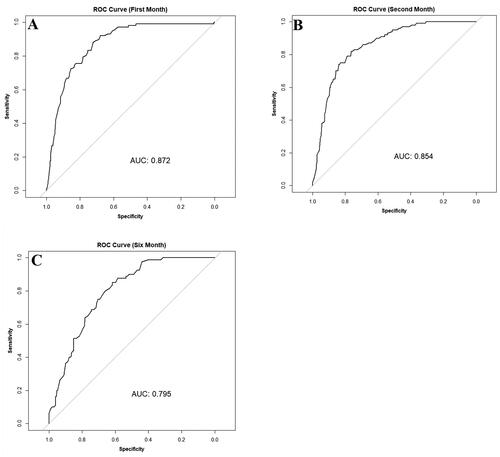
In patients with initially normal total cholesterol, 83.71% maintained normal levels after one month (normal-normal), while 16.29% developed grade 1 abnormalities (normal-grade 1). An independent t-test demonstrated an increase in baseline mean across patients who developed grade 1 abnormalities compared to patients with persistent normal levels (p < 0.001, ). Chi-square analysis revealed an association between baseline quartile and change at all timepoints (p < 0.001). For instance, among patients initially in the fourth quartile with normal baseline cholesterol levels (172.0 mg/dL to 199.5 mg/dL), 56.45% developed grade 1 abnormalities after one month, compared to 21.51%, 3.91%, and 0.57% in the third (156.0 mg/dL to 172.0 mg/dL), second (137.0 mg/dL to 156.0 mg/dL), and first (83.1 mg/dL to 137.0 mg/dL) quartiles, respectively. ROC (Receiver Operating Characteristic) analysis indicated a baseline cholesterol cutoff of 163.9 with a specificity of 72.33% and sensitivity of 88.24% for detecting grade 1 abnormalities at the one month (Youden’s index = 0.61, ). In the second and sixth months, cutoff values of 165.95 mg/dL and 156.4 mg/dL corresponded to specificities of 76.38% and 61.74%, sensitivities of 82% and 85%, and Youden’s indices of 0.58 and 0.47, respectively (,C)).
The Influence of baseline triglycerides levels on follow-up abnormalities
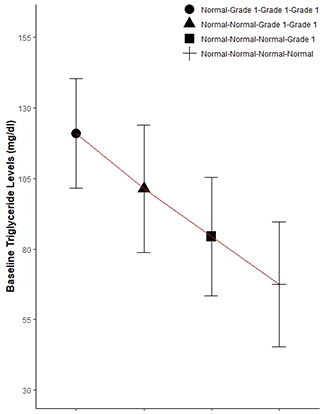
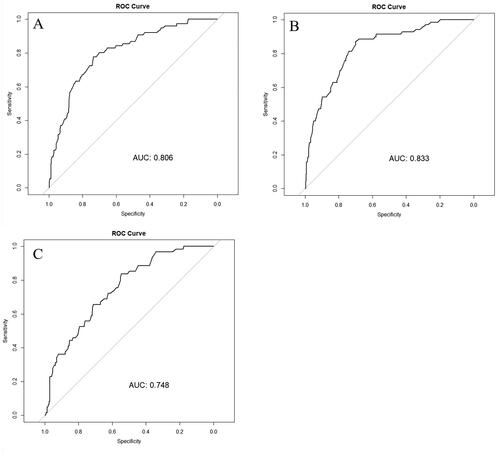
Among patients initially with normal triglycerides levels, 88.57% maintained normal levels after one month (normal-normal), while 11.43% developed grade 1 abnormalities (normal-grade 1). An independent t-test demonstrated an increase in baseline mean among patients with grade 1 abnormalities compared to those with persistent normal levels (p < 0.001, ). Chi-square analysis revealed an association between baseline quartile and abnormality development at all timepoints (p < 0.001). For instance, among patients initially in the fourth quartile with normal baseline triglycerides levels (93.5 mg/dL to 149.0 mg/dL), 30.67% developed grade 1 abnormalities after one month, compared to 9.26%, 4.62%, and 1.8% of patients in the third (70.5 mg/dL to 93.0 mg/dL), second (55.0 mg/dL to 70.0 mg/dL), and first (18.0 mg/dL to 54.3 mg/dL) quartiles, respectively. ROC analysis indicated a baseline triglycerides cutoff of 85.5 mg/dL with a specificity of 73.68% and sensitivity of 77.63% for detecting grade 1 abnormalities at one month (Youden’s index =0.51; ). In the second and sixth months, cutoff values of 81.4 mg/dL and 71.5 mg/dL corresponded to specificities of 69.86% and 54.78%, sensitivities of 87.1% and 83.61%, and Youden’s indices of 0.57 and 0.38, respectively (,C)).
Immune cell ratios and systemic inflammatory index change during treatment course
Baseline mean values of NLR, PLR, and SII were 2.02 ± 1.26, 9.14 ± 4.75, and 556.65 ± 402.93, respectively. Paired t-test analysis showed a decrease in NLR and SII values after six months (p = 0.012 and p = 0.021, respectively). PLR showed a trend toward reduction close to statistical significance (p = 0.051) ().
Discussion
Oral isotretinoin is a very effective and widely used medication (Citation7,Citation12). However, it may cause laboratory irregularities, particularly affecting lipid panels, transaminases, and hematologic parameters (Citation2–4,Citation7). Given its prolonged use over several months, regular laboratory assessments are necessary for patient safety (Citation6). Despite prior monthly monitoring (Citation12), recent studies have questioned the necessity of frequent lab monitoring (Citation13–20), as abnormalities are usually mild and have minimal impact on management (Citation13,Citation14). Although there is no established consensus regarding the frequency of these assessments (Citation6,Citation13,Citation19,Citation21–24), regular lab monitoring continues to be a standard practice among many physicians (Citation12,Citation13).
Multiple studies, including a 2006 retrospective study on 13772 patients (Citation17) and a 2016 study on 515 patients (Citation19), found mild and infrequent CBC changes during isotretinoin treatment (Citation13,Citation16,Citation17,Citation19). Guidelines from the American, European (Citation21), British National Formulary (BNF) (Citation25), and Electronic Medicines Compendium (EMC) (Citation26) do not recommend CBC monitoring while on isotretinoin. However, the British Association of Dermatologists (BAD) suggests checking CBC after 4–6 weeks of initiation and every 3 months thereafter (Citation27).
Hansen et al. suggested that for a healthy patient with a normal baseline, monitoring the lipid panel and transaminases after 2 months of isotretinoin is sufficient unless risk factors or an abnormal baseline are present, as supported by a study on 515 patients (Citation19). Shah et al. recommended monitoring 2–3 months after the peak isotretinoin dose and at course completion, based on a 2018 retrospective analysis of 903 patients where elevations resolved post-treatment (Citation23). Park et al. advised checking transaminases and triglycerides every six months after reaching peak dose, with LDL and total cholesterol checked after 1 and 2 months of isotretinoin. Based on a 2022 South Korean study on 30 patients, this study suggested repeating the monitoring throughout the isotretinoin course (Citation6), in contrast to other studies that recommended only repeating labs if there is an abnormal baseline or risk factors (Citation19,Citation23). American guidelines recommend periodic liver function and lipid panel checks (Citation21). European, BNF, and EMC recommend monitoring one month after initiation and every 3 months after that (Citation21,Citation25,Citation26). BAD recommends monitoring after 4–6 weeks, then every 3 months (Citation27).
A recent systematic review of 125 papers was conducted and recommended the avoidance of extensive lab monitoring in healthy young individuals due to the rarity of serious laboratory changes while on isotretinoin, and monitoring should be individualized based on the patient’s specific risk factors (Citation20).
Some studies questioned the necessity of baseline lab testing before isotretinoin initiation (Citation14) while others, including American, European, and British guidelines, recommend baseline lipid profile and liver function checks (Citation6,Citation21,Citation23,Citation25–27). However, checking the baseline CBC is only recommended by BAD guidelines (Citation27). Interestingly, we found a very important correlation between the baseline serum lipids and the possibility of lab abnormalities while on the isotretinoin treatment course. We identified baseline cutoffs (cholesterol: 163.9 mg/dl, triglycerides: 85.5 mg/dL) with high specificity and sensitivity for detecting grade 1 abnormalities at the one-month follow-up, Which is a novel contribution to the field.
Acne’s multifactorial pathogenesis involves pilosebaceous unit inflammation (Citation28), with isotretinoin playing a crucial anti-inflammatory role (Citation7). Recent studies have explored isotretinoin correlation with inflammatory markers (Citation8–10). Two 2013 and 2015 retrospective studies (110 and 112 patients, respectively) found no significant NLR and PLR changes post-isotretinoin (Citation9,Citation10). In contrast, 2021 and 2022 case-control studies (190 patients/66 controls and 156 patients/100 controls, respectively) reported reduced NLR but insignificantly reduced PLR post-isotretinoin (Citation8,Citation11). Another 120-patient retrospective study showed decreased NLR and PLR post-isotretinoin (Citation29). SII, proposed as a more useful inflammatory indicator, decreased in two case-control studies (190 patients/66 controls and 156 patients/100 controls) (Citation8,Citation11). Our study found reduced NLR and SII after six months of isotretinoin, with PLR decreasing, though not reaching statistical significance.
In conclusion, most observed lab abnormalities were mild, suggesting extensive lab monitoring is unnecessary, aligning with prior studies (Citation13–20). We stress the importance of baseline lipid panel checks, having identified baseline cutoffs for cholesterol and triglycerides as effective risk stratification methods for isotretinoin acne patients. Additionally, we emphasize isotretinoin’s anti-inflammatory role, confirmed by NLR and SII decline. We hypothesize that lowering these parameters may influence treatment duration and serve as predictors for acne relapse post-treatment. Nevertheless, further studies are warranted to either validate or disprove these hypotheses.
Limitations
As with other retrospective studies, ours has limitations, such as missed individual labs and fixed follow-up visits for some patients. Being a single-center study, our findings may not fully represent the general Jordanian population. Therefore, we recommend multi-centric population-based studies for broader insights.
Ethical approval
The Institutional Review Board of Jodan University Hospital approved this study (IRB No. 95/2023). A copy of the approval letter is attached to the submission.
Disclosure statement
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Ahmad HM. Analysis of clinical efficacy, side effects, and laboratory changes among patients with acne vulgaris receiving single versus twice daily dose of oral isotretinoin. Dermatol Ther. 2015;28(3):1–7. doi:10.1111/dth.12213.
- Alajaji A, Alrawaf FA, Alosayli SI, et al. Laboratory abnormalities in acne patients treated with oral isotretinoin: a retrospective epidemiological study. Cureus. 2021;13(10):e19031. doi:10.7759/cureus.19031.
- Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglycerides and liver aminotransferases. An Bras Dermatol. 2012;87(3):382–387. doi:10.1590/s0365-05962012000300005.
- Kızılyel O, Metin MS, Elmas ÖF, et al. Effects of oral isotretinoin on lipids and liver enzymes in acne patients. Cutis. 2014;94(5):234–238. https://pubmed.ncbi.nlm.nih.gov/25474452/
- Macek C. Synthetic vitamin a analogue (isotretinoin) awaiting approval for cystic acne therapy. JAMA. 1982;247(13):1800–1801.
- Park YJ, Shin HY, Choi WK, et al. Optimal laboratory testing protocol for patients with acne taking oral isotretinoin. World J Clin Cases. 2023;11(11):2435–2442. doi:10.12998/wjcc.v11.i11.2435.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1(3):162–169. doi:10.4161/derm.1.3.9364.
- Turan Ç, Metin N. A novel inflammatory marker in the follow-up of moderate-to-Severe acne vulgaris administered isotretinoin: systemic immune-Inflammation index (SII). Curr Health Sci J. 2022;48(1):63–67. doi:10.12865/CHSJ.48.01.09.
- Ataseven A, Kurtipek G, Ozturk P. Neutrophil lymphocyte ratio in patients receiving isotretinoin for acne vulgaris. Med Sci | Int Med J. 2014;3(1):1026. doi:10.5455/medscience.2013.02.8101.
- Seçkin HY, Baş Y, Takçı Z, et al. Effects of isotretinoin on the inflammatory markers and the platelet counts in patients with acne vulgaris. Cutan Ocul Toxicol. 2016;35;(2):89–91. doi:10.3109/15569527.2015.1021927.
- Cosansu NC, Yuksekal G, Turan U, et al. Investigation of systemic immune-inflammation index and systemic inflammation response index as an indicator of the anti-inflammatuary effect of isotretinoin in patients with acne vulgaris. Cutan Ocul Toxicol. 2022;41(2):174–178. doi:10.1080/15569527.2022.2081700.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. doi:10.1016/j.jaad.2015.12.037.
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82(1):72–79. doi:10.1016/j.jaad.2019.06.025.
- Tkachenko E, Sharma P, Mostaghimi A. Abnormal baseline lab results rarely lead to treatment modification for patients on isotretinoin. Dermatology. 2020;236(6):517–520. doi:10.1159/000505451.
- Opel D, Kramer ON, Chevalier M, et al. Not every patient needs a triglyceride check, but all can get pancreatitis: a systematic review and clinical characterization of isotretinoin-associated pancreatitis. Br J Dermatol. 2017;177(4):960–966. doi:10.1111/bjd.15207.
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne. JAMA Dermatol. 2016;152(1):35–44. doi:10.1001/jamadermatol.2015.3091.
- Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016–1022. doi:10.1001/archderm.142.8.1016.
- Altman RS, Altman LJ, Altman JS. A proposed set of new guidelines for routine blood tests during isotretinoin therapy for acne vulgaris. Dermatology. 2002;204(3):232–235. doi:10.1159/000057887.
- Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75(2):323–328. doi:10.1016/j.jaad.2016.03.019.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187(6):857–865. doi:10.1111/bjd.21840.
- Dessinioti C, Zouboulis CC, Bettoli V, et al. Comparison of guidelines and consensus articles on the management of patients with acne with oral isotretinoin. J Eur Acad Dermatol Venereol. 2020;34(10):2229–2240. doi:10.1111/jdv.16430.
- Öktem A, Hayran Y, Arı E, et al. Minimize the regular laboratory monitoring during the systemic isotretinoin treatment: data of 704 patients with acne vulgaris. J Dermatolog Treat. 2019;30(8):813–817. doi:10.1080/09546634.2019.1591578.
- Shah R, Kroshinsky D. Re-evaluating the need for routine laboratory monitoring in patients taking isotretinoin: a retrospective analysis. J Am Acad Dermatol. 2021;85(2):504–506. doi:10.1016/j.jaad.2018.10.005.
- Ben-Shoshan D, Gomolin A, Litvinov IV, et al. Time to change guidelines for laboratory monitoring during isotretinoin treatment. J Cutan Med Surg. 2020;24(1):92–93. doi:10.1177/1203475419879882.
- National Institute for Health and Care Excellence. British National Formulary (BNF). https://bnf.nice.org.uk (last accessed 15 November 2023).
- Electronic Medicines Compendium. Roaccutane 20 mg soft capsules.https://www.medicines.org.uk/emc/product/6470/smpc (last accessed 15 November 2023).
- Goodfield MJD, Cox NH, Bowser A, et al. Advice on the safe introduction and continued use of isotretinoin in acne in the U.K. 2010. Br J Dermatol. 2010;162(6):1172–1179. doi:10.1111/j.1365-2133.2010.09836.x.
- Tanghetti EA. The role of inflammation in the pathology of acne. J Clin Aesthet Dermatol. 2013;6(9):27–35.
- Kutlu Ö. Effect of isotretinoin treatment on the inflammatory markers in patients with acne vulgaris: can monocyte/HDL be a new indicator for inflammatory activity of isotretinoin treatment? Cutan Ocul Toxicol. 2020;39(1):67–70. doi:10.1080/15569527.2019.1701485.