Abstract
Aim
This study aimed to compare the efficacy and safety of excimer laser (EL)-based combination regimens in improving repigmentation.
Methods
A comprehensive search was conducted in PubMed, Web of Science, Cochrane Library, and Embase on July 1, 2023, to include randomized controlled trials of EL combination treatments for vitiligo that met the criteria. The primary outcome measure was a repigmentation rate ≥ 75%, and the secondary outcome measures were a repigmentation rate of ≤ 25% and adverse events.
Results
Eleven studies involving 348 patients were included. Network Meta-Analysis showed that EL combined with antioxidants (SUCRA = 98.8%), EL combined with calcipotriol (SUCRA = 59.8%) and EL combined with tacalcitol (SUCRA = 59.6%) were the three optimal interventions achieving repigmentation rates ≥ 75%. EL alone (SUCRA = 77.6%), EL combined with tacalcitol (SUCRA = 61.7%) and EL combined with antioxidants (SUCRA = 57.2%) were the three interventions with the highest rates of treatment failure. Adverse events in all groups mainly included erythema, burning sensation and hyperpigmentation. Based on the results of the current study, EL combination therapies were safe with mild adverse events.
Conclusion
EL combined with antioxidants was the preferred regimen for vitiligo, whereas EL alone was the regimen with the highest rate of treatment failure in vitiligo.
Introduction
Vitiligo is an acquired skin disorder caused by a loss of pigment. It is characterized by well-demarcated depigmented patches resulting from the functional loss or decreased number of melanocytes in the mucosa, epidermis and other tissues (Citation1). Vitiligo can occur in people of any age or gender and affects 0.5%–2% of the global population (Citation2,Citation3). The prevalence of vitiligo is estimated to be 0.76%–1.11% in the United States, 1.6% in Europe, and 0.5% in Japan (Citation4,Citation5). Although vitiligo may not endanger the patient’s life, it may be stigmatizing due to the color difference between the lesion and normal skin, thereby causing psychological disturbance, increasing the risk for psychiatric disorders and social stress, and seriously affecting the quality of life in patients (Citation6,Citation7). In addition, vitiligo will impose a substantial social burden, including direct costs spent on vitiligo healthcare services and indirect costs due to absenteeism (Citation8).
The clinical treatment of vitiligo is challenging. Common treatments currently available include pharmacotherapy, physiotherapy and surgery. Psoralen plus ultraviolet A (PUVA), narrow-band ultraviolet B (NB-UVB) and excimer laser (EL) are main types of pharmacotherapy for vitiligo (Citation9). PUVA is an early pharmacotherapy option used for vitiligo patients. It converts psoralen compounds into oxidizing chemical products through UVA light to inhibit immune function and stimulate melanocyte proliferation and pigmentation (Citation10,Citation11). Although PUVA is fairly effective, its unavoidable side effects including nausea, vomiting, headache, depression and phototoxicity and its potential risk for skin cancer have led to a decrease in its clinical use (Citation12). Compared with PUVA, NB-UVB has the advantages including no use of photosensitizers, fewer adverse reactions and a wider target population (Citation13,Citation14), so it has surpassed PUVA as a first-line treatment option for vitiligo (Citation12). A net meta-analysis (NMA) has been conducted to compare NB-UVB combination therapy regimens for vitiligo, so as to provide a recommendation for NB-UVB therapy for vitiligo (Citation15). Like NB-UVB, EL may be immunomodulatory and immunosuppressive to promote and maintain repigmentation in vitiligo (Citation16). Several studies have shown that the efficacy of EL in treating vitiligo outperforms that of NB-UVB, with the advantages including faster repigmentation, less frequent treatment, lower cumulative doses, no effect on normal skin, and milder side effects, so EL is more competitive for the treatment of localized vitiligo, and it can effectively avoid irradiation to the skin around the lesion (Citation9,Citation16,Citation17). In recent years, many vitiligo researchers have focused on EL combination therapies involving calcineurin inhibitors (Citation18,Citation19), antioxidants (Citation20), glucocorticoids (Citation21), photosensitizer (Citation22) and platelet-rich plasma (PRP) (Citation23). Combination therapies mainly act synergistically to improve efficacy on the one hand, and to reduce the total dose and duration of treatment on the other hand, so as to achieve earlier and greater repigmentation (Citation24).
Currently, there are still no studies using all the data on EL combination therapies for vitiligo to create a network for direct and indirect comparisons for multiple analyses of EL combination therapies for vitiligo, although Bae et al. (Citation17) have only routinely made a direct head-to-head comparison of EL combination therapies for vitiligo. Therefore, based on the published literature, we directly and indirectly compared the efficacy of current EL-based combination regimens in improving repigmentation via a systematic evaluation and NMA.
Materials and methods
The NMA followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) as a guideline and its requirements for NMAs. The study protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42023461012).
Search strategy
Two researchers (ChanXiu Li and YaPing Wang) independently searched PubMed, Web of Science, Cochrane Library and Embase for studies on EL combination therapies for vitiligo published from their establishment to July 1, 2023, with the following subject headings and free-text words and their combinations as search terms: "Excimer Laser*", "Krypton Chloride Laser*", "KrCl Laser*", "Xenon Chloride Laser*", "XeCl Laser*", "ArF Laser*", "Argon Fluoride Laser*", "excimer laser", "EC-5000", "XTRAC" "Vitiligo". The search strategy is detailed in the Supplemental Table S1. At the same time, we conducted a secondary search for references in relevant published articles or systematic reviews to ensure the comprehensiveness of the retrieved literature as much as possible.
Inclusion and exclusion criteria
Studies meeting the following established criteria will be included in this study (Citation1): study subjects: patients with vitiligo aged ≥ 18 years (Citation2); interventions: EL alone or EL combined with topical pharmacotherapy (Citation3); study type: randomized controlled trials (RCTs) (Citation4); outcome measures: the primary outcome measure is the treatment success rate, i.e., a repigmentation rate ≥ 75%, and the secondary outcome measures are the treatment failure rate, i.e., a repigmentation rate ≤ 25% (Citation17), and adverse events (including erythema, burning sensation and hyperpigmentation); and (Citation5) language: English.
Studies meeting the following criteria will be excluded (Citation1): case reports, animal or cellular experiments, meta-analyses, reviews, letters, and meeting abstracts (Citation2); duplicate studies (Citation3); studies with missing data (Citation4); unavailable full texts.
Data extraction
The retrieved literature was imported into Endnote software. Firstly, two researchers (Yue Hu and ZengYi Mu) independently screened the titles and abstracts of the included literature with reference to the inclusion and exclusion criteria, and then read the full texts for second screening. Two researchers used Excel 2019 to independently extract data from the included literature, including (Citation1) the name of the first author, the year of publication, the main country in which the study was conducted, and the blinded design; and (Citation2) the intervention information including the intervention name, sample size, mean age, male-to-female sex ratio, treatment duration, EL treatment site, follow-up time, and outcome measures. All disagreements that arose between the two researchers during the process of literature screening, data extraction and sorting were resolved through discussion with the third researcher (Lei Shi).
Quality assessment
Two researchers (Xiao Sun and XinYue Wang) independently assessed the risk of bias of the original studies using a Cochrane risk of bias assessment tool (RoB2.0) in 6 domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, bias in selection of the reported result, and overall bias. For each study, the six domains involved were assessed as "high risk", "low risk" and "unclear risk". If disagreements arose between the two researchers during this process, they would be resolved through a joint discussion with the third researcher (Lei Shi). The assessment results are presented in a risk of bias graph.
Statistical analysis
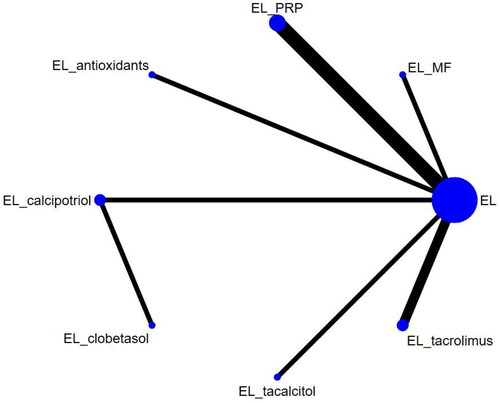
NMA was performed using Stata15.1 and R4.2.1 software. The repigmentation rate was expressed as a risk ratio (RR), and the 95% confidence interval (CI) was used to express the results of statistical analysis. Given the heterogeneity among the trials, a Bayesian stratified random-effects model was first fitted for multiple comparisons of different therapeutic regimens for vitiligo (Citation25). On the one hand, all calculations and graphics were generated with Stata 15.1 and R4.2.1 software. Based on the likelihood theory and some prior assumptions, Markov Chain Monte Carlo (MCMC) simulations were performed using R4.2.1 software for Bayesian inference to run 50,000 iterations including the first 20,000 ones for burn-in to remove any potential influence from the initial starting values, so as to investigate the posterior distribution of query nodes (Citation26). The node-splitting method was used to assess local inconsistency in results with closed loops. Network diagrams were plotted to present the direct relationships among different interventions, with the diameter of dots being proportional to the sample size in the interventions, and the thickness of lines being proportional to the number of literature references for direct comparison between interventions. At the same time, a comparison-correction funnel plot was drawn to test for the presence of a small-sample effect, i.e., potential publication bias (Citation27). In addition, the surfaces under the cumulative ranking curve (SUCRA) for the different interventions were calculated and ranked with values ranging from 0 to 1, of which the larger one indicated better efficacy of the intervention (Citation28). A ranking table was created to compare each pair of interventions in each outcome.
Results
Literature search and screening results
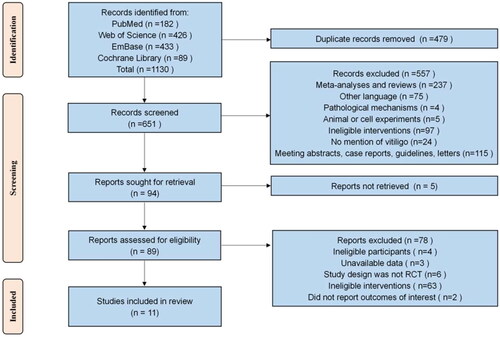
The initial search of the four major databases yielded a total of 1130 studies, and no additional eligible studies were identified through the secondary search of references in relevant published articles or systematic reviews. After the removal of 479 duplicates, 557 studies were rejected by an initial review of titles and abstracts. The full texts of the remaining 94 studies were then searched, and the full texts of 5 of them were not available. Next, 89 studies with full texts available were evaluated according to the inclusion and exclusion criteria, and 78 of them were excluded. Finally, a total of 11 studies were included in the systematic evaluation and NMA. The screening process is detailed in .
Baseline characteristics of included studies
A total of 348 patients with vitiligo were recruited in 11 RCTs conducted in Egypt (Citation20,Citation29,Citation30), Thailand (Citation21,Citation31), China (Citation23,Citation32), Iran (Citation33), Italy (Citation34), France (Citation35) and the United States (Citation36), including 133 males and 215 females aged 4-91 years, and sample sizes ranging from 8 to 45 participants in the studies. The specific characteristics of the studies are presented in . Of the studies, 3 investigated EL combined with PRP (Citation23,Citation29,Citation30), while 2 investigated EL combined with calcipotriol (CAL) (Citation31,Citation33). Besides, EL combined with clobetasol (CLO) (Citation31), EL combined with mometasone furoate (MF) (Citation21), EL combined with antioxidants (AO) (Citation20), EL combined with hydrocortisone 17-butyrate cream (HB) (Citation34) and EL combined with tacalcitol (TAC) (Citation32) were investigated in 1 study, respectively, and 2 investigated EL combined with tacrolimus (TRL) (Citation35,Citation36). Meanwhile, the treatment duration was ≥4 months in a study by Elsaadany et al. (Citation29), 4 months in studies by Khattab et al. (Citation30) and Ghalamkarpour et al. (Citation33), 10 weeks in a study by Kawalek et al. (Citation36), and 3 months in all the remaining studies. In addition, the follow-up time was 4 months in 2 studies (Citation33,Citation34), 6 months in 1 study (Citation36), and 3 months in all the remaining studies.
Table 1. Characteristics of studies.
Risk of bias assessment
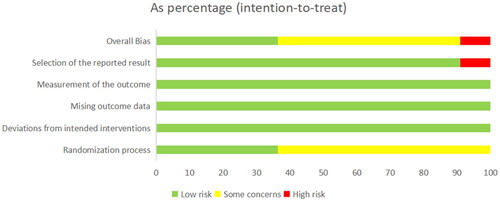
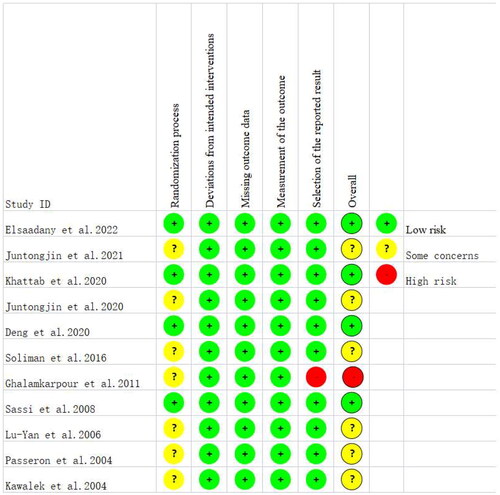
Risk of bias in the 11 studies included was assessed using RoB2.0. The results are detailed in and Citation3. shows judgments for all domains in percentage form. displays domain-specific risk of bias in all the included studies. Different colors (red, green, yellow) and symbols ("−", "+", "?") in the figures denotes "high risk", "low risk" and "unclear risk", respectively. Of the 11 studies included, four were at low overall risk of bias (Citation23, Citation29,Citation30,Citation34). In terms of bias arising from the randomization process, seven studies were assessed to be at unclear risk due to unclear random assignment or allocation concealment (Citation20,Citation21,Citation31–33,Citation35,Citation36), and the remaining 4 studies were at low risk. In terms of bias in selection of the reported result, 1 study was assessed to be at high risk due to selective reporting (Citation33), and the remaining 10 studies were at low risk. In terms of bias due to deviations from intended interventions, all the studies were assessed to be at low risk. In terms of bias due to missing outcome data and bias in measurement of the outcome, all the studies were assessed to be at low risk.
Network analysis results
Network diagrams
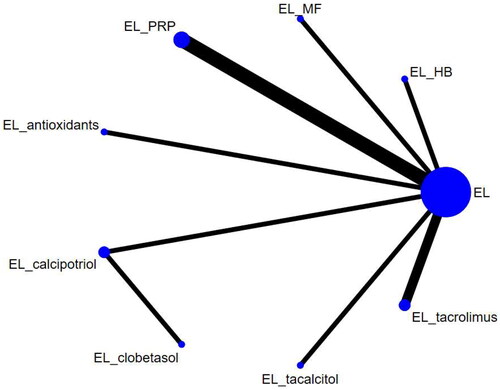
The 11 studies included covered 9 different interventions. Network diagrams for the different interventions are shown in and .
Repigmentation rate ≥ 75%
The repigmentation rate ≥ 75% was reported in 11 studies, including 9 interventions. The results showed that in terms of the repigmentation rate ≥ 75%, EL combined with AO was superior to EL alone (RR = 176871883.21, 95% CI. 15.41, 1.58707326992159e + 27). EL combined with PRP was superior to EL combined with CLO (RR = 88314522535155.5, 95% CI: 1.07, 9.06479759418698e + 44). EL combined with CAL (RR = 0, 95% CI: 0, 0.84), EL combined with CLO (RR = 0, 95% CI: 0, 0), EL combined with HB (RR = 0, 95% CI: 0, 0.26), EL combined with MF (RR = 0, 95% CI: 0, 0.27), EL combined with PRP (RR = 0, 95% CI: 0, 0.42), EL combined with TAC (RR = 0, 95% CI: 0, 0.63) and EL combined with TRL (RR = 0, 95% CI: 0, 0.28) were inferior to EL combined with AO. EL combined with CLO (RR = 0, 95% CI: 0, 0.37) was inferior to EL combined with CAL. All the differences above were statistically significant. There were no statistically significant differences between the other pairwise interventions ().
Table 2. Network meta-analysis of the repigmentation rate (≥75%).
The SUCRA results showed that in terms of the repigmentation rate ≥ 75%, the probabilities of efficacy of EL alone and combination therapies were ranked as EL combined with AO (98.8%) > EL combined with CAL (59.8%) > EL combined with TAC (59.6%) > EL combined with PRP (58.8%) > EL combined with TRL (52.4%) > EL combined with HB (47.9%) > EL combined with MF (45.0%) > EL alone (25.3%) > EL combined with CLO (2.5%), of which EL combined with AO, EL combined with CAL and EL combined with TAC were the three optimal interventions ( and ).
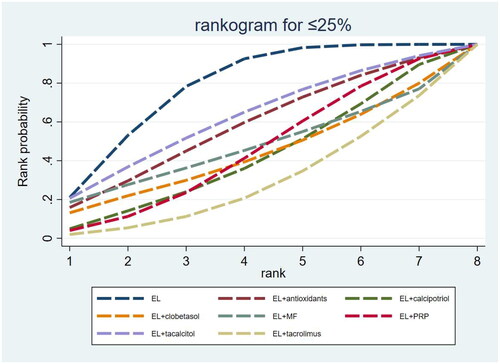
Figure 6. Surfaces under the cumulative ranking curve (SUCRA) probability line chart for the repigmentation rate ≥ 75%. EL: excimer laser; HB: hydrocortisone 17-butyrate cream; MF: mometasone furoate; PRP: platelet-rich plasma.
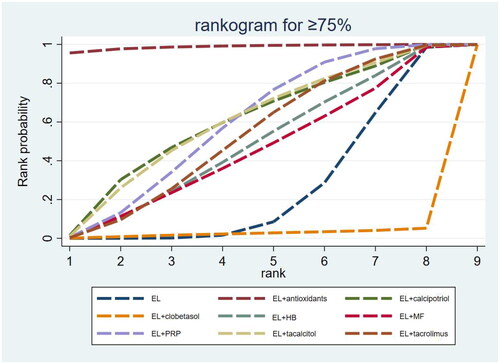
Table 3. Surfaces under the cumulative ranking curve ranking for repigmentation rates (≥75% and ≤25%).
Repigmentation rate ≤ 25%
The repigmentation rate ≤ 25% was reported in 10 studies, including 8 interventions. There were no statistically significant differences between all the pairwise interventions ().
Table 4. Network meta-analysis of the repigmentation rate (≤25%).
The SUCRA results showed that in terms of the repigmentation rate ≤ 25%, the probabilities of failure of EL alone and combination therapies in descending order were ranked as EL alone (77.6%) > EL combined with TAC (61.7%) > EL combined with AO (57.2%) > EL combined with MF (46.5%) > EL combined with PRP (44.5%) > EL combined with CLO (42.7%) > EL combined with CAL (41.3%) > EL combined with TRL (28.6%), of which EL alone, EL combined with TAC and EL combined with AO were the three interventions with the highest rates of treatment failure ( and ).
Adverse events
Of the 11 studies, 2 (18.2%) reported no adverse events (Citation20,Citation23), 3 (27.3%) did not report detailed information on adverse events (Citation21,Citation31,Citation32), and 6 (54.5%) reported 7 adverse events in detail (Citation29,Citation30,Citation33–36). The interventions in the 6 studies included EL alone (Citation29,Citation30,Citation33–36), EL combined with PRP (Citation29,Citation30), EL combined with CAL (Citation33), EL combined with HB (Citation34), and EL combined with TRL (Citation35,Citation36). Of the 6 studies, each reported erythema, 1 reported burning sensation (Citation29), 2 reported hyperpigmentation (Citation29,Citation34), 2 reported pain (Citation30,Citation35), 1 reported photosensitivity (Citation33), 1 reported bullae (Citation35), and 1 reported vesicles (Citation33). The incidence of burning sensation (9.8%) and hyperpigmentation (23.2%) was greatest in patients treated with EL combined with PRP. The incidence of photosensitivity (0.4%) and vesicles (0.4%) was greatest in patients treated with EL alone. The incidence of erythema (100%), pain (15.2%) and bullae (6.1%) was greatest in patients treated with EL combined with TRL. The greatest incidence of erythema in patients treated with EL combined with TRL was possibly related to the different definitions of erythema in different studies. Kawalek, et al. scored erythema according to its severity, whereas the remaining studies showed the presence or absence of erythema. Adverse reactions are detailed in .
Table 5. Adverse events reported in included studies.
Publication bias
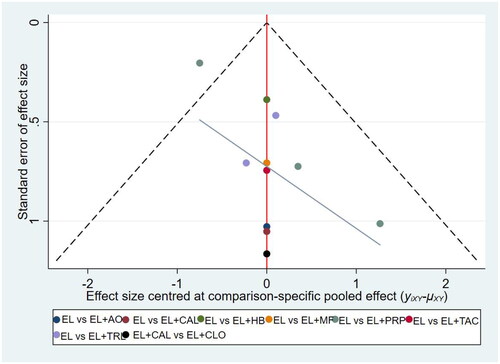
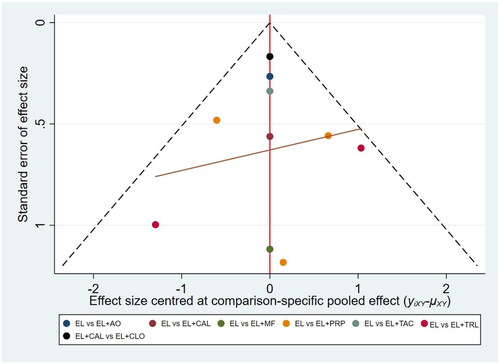
Publication bias for outcome measures was assessed using funnel plots with points of different colors representing a comparison of two interventions. In the funnel plot for the repigmentation rate ≥ 75%, some points are located above the funnel plot and not symmetrically or evenly distributed, suggesting that there might be some publication bias (). In the funnel plot for the pigmentation rate ≤ 25%, all the points were evenly and symmetrically distributed on both sides of the funnel plot, suggesting that there might be no publication bias ().
Discussion
To our knowledge, this is the first NMA comparing the efficacy of different EL combination therapies for vitiligo. The NMA used the most recent data from a total of 11 eligible RCTs. Our findings showed that EL combined with AO was the most effective intervention in terms of the repigmentation rate ≥ 75%, and EL alone was the intervention with the highest rate of treatment failure in vitiligo.
Summary of key findings
EL combined with AO was the most effective intervention for vitiligo in terms of the repigmentation rate ≥ 75%. Vitiligo is most characterized by the loss of melanocytes in the affected epidermis. Oxidative stress is considered an important cause of the loss of melanocytes and plays an extremely important role in the occurrence and development of vitiligo (Citation37). When the body is exposed to stimuli from multiple types of harmful factors, such as adverse environmental factors, and the capacity to scavenge oxides is less than the oxidation capacity, then the body will produce excess reactive oxygen species (ROS) and other highly reactive molecules, a process known as oxidative stress. Oxidative stress is a pathological condition that is in essence a disturbance of redox homeostasis characterized by a persistent imbalance between antioxidants and pro-oxidants (Citation38). Recent studies have confirmed the existence of redox imbalance in serum from patients with vitiligo and their melanocytes in affected skin (Citation39,Citation40). ROS include carboxyl free radicals and hydrogen peroxide (H2O2). Jian et al. (Citation41) found that melanocytes were hypersensitive to H2O2-induced oxidative damage, thereby leading to a decreased level of expression of the antioxidant enzyme heme oxygenase-1 (HO-1) and redox imbalance, and in addition, the HO-1 level was significantly lower in patients with vitiligo compared with healthy controls. Therefore, oxidative stress may reduce the level of antioxidant enzyme expression and increase melanocyte death. Zedan et al. (Citation42) found that the level of activity of the antioxidant enzyme glutathione peroxidase (Gpx) was significantly lower in patients with vitiligo compared with healthy controls. Mitochondria are not only a major source of ROS but also control cellular energy supply, necrosis and apoptosis. They are inextricably linked to cells and key to oxidative stress-mediated apoptosis of melanocytes. On the one hand, a study by Kang et al. (Citation43) showed that oxidative stress triggered by excess ROS was able to induce mitochondrial dysfunction and would further induce the production of more ROS. Sustained oxidative stress will not only lead to lipid and protein peroxidation but also damage DNA. Melanocyte DNA may enter the irreversible cell cycle arrest, i.e., cellular senescence, after undergoing damage caused by persistent oxidative stress stimulation (Citation44). On the other hand, oxidative stress can also in turn expose melanocytes to immune damage through the synthesis of toxic melanin intermediates, which release catecholamines. In addition, oxidative stress can upregulate the expression of a series of chemokines including C-X-C motif chemokine ligand (CXCL) 10 and CXCL16, which drive T cells to exert a killing effect on melanocytes. Therefore, antioxidant strategies play an important role in controlling the course of vitiligo. The benefits of AO for vitiligo treatment have been shown in consensuses on the diagnosis and treatment of vitiligo or guidelines for vitiligo treatment developed by some countries. The relevant consensus developed by China recommends the combination of phototherapy with AO for better efficacy (Citation45). The Brazilian Society of Dermatology (SBD) recommends the combination of phototherapy with AO for the treatment of unstable vitiligo (Citation46). The European guideline considers that AO can increase the efficacy of phototherapy (Citation47). Studies by Soliman et al. (Citation20) and Leone et al. (Citation48) confirmed that EL combined with AO was more effective than EL alone in the treatment of vitiligo, with significantly faster repigmentation and higher patient satisfaction. These studies suggest that the combination of AO with EL can not only shorten the duration of EL treatment but also reduce the occurrence of potential adverse events to a greater extent. Although more and more studies confirmed the positive effect of AO on vitiligo (Citation49,Citation50), the frequency of AO application in clinical practice is lower than the frequency of use of drugs including glucocorticosteroids and calcineurin inhibitors, possibly for two reasons. On the one hand, clinicians may have certain deficiencies in their knowledge of AO, and various guidelines recommend AO combined with phototherapy but do not mention specific dosing regimens of AO, so clinicians are uncertain about the efficacy, specific dosing regimens and side effects of AO. On the other hand, clinicians also need to consider patients’ feelings, because vitiligo patients may be afraid of AO and reject its use due to the traditional idea of "whitening effect” of AO (Citation51). Therefore, it is hoped that relevant guidelines will be issued to specifically guide clinicians in the use of AO and to provide patients with common knowledge to some extent. In addition, due to the limited number of studies included in this study, there was only one literature reference on EL combined with AO, which may have a certain impact on the stability and feasibility of the conclusion. Therefore, it is required to be cautious about drawing the conclusion that EL combined with AO was the most effective intervention for vitiligo.
EL alone was the intervention with the highest rate of treatment failure in vitiligo in terms of the repigmentation rate ≤ 25%. Since 2002 when EL was first reported to be used for repigmentation of vitiligo, studies on EL have been carried out in full swing (Citation52,Citation53). Similar to NB-UVB, EL is able to not only induce T-cell apoptosis and effectively suppress inflammation, but also promote melanin production and melanocyte proliferation, thereby inducing pigment regeneration (Citation54). Compared with conventional phototherapy, EL treatment for vitiligo has the advantages including better efficacy, no impact on normal skin, faster onset of action, and a more affordable option (Citation17). Although EL alone has good efficacy, it still cannot fully meet the needs for clinical treatment, and it is difficult to develop color in EL alone especially for lesions in regions insensitive to UV treatment, including bony prominences and distal limbs. To enhance the role of EL in the treatment of vitiligo, topical medications are often widely used clinically based on EL to exert a synergistic effect (Citation24).
We were unable to perform an NMA of adverse events caused by the interventions due to limited raw data from the included studies, some of which only described adverse events and did not give their details. In addition to the specific adverse events mentioned previously, some tolerable adverse events including capillary dilation (Citation31), dryness and pruritus (Citation32) were described in the included studies. Taken together, EL combination therapies were safe with mild adverse events. How to select an appropriate treatment modality and treatment frequency for patients with vitiligo, to be alert to the occurrence of serious adverse effects and to balance the benefits and risks in clinical treatment are still issues worthy of elaboration and exploration by the researchers.
Clinical significance
With more and more studies recognizing the role of EL in inducing pigmentation, EL combined with pharmacotherapy for patients with vitiligo in recent years has been shown to be an effective treatment (Citation29,Citation31), Meanwhile, there is still no NMA evaluating the efficacy of EL combination therapies for vitiligo, although several systematic evaluations, meta-analyses and NMAs have been conducted in the past to evaluate the efficacy of phototherapy combination therapies for vitiligo, and most of these studies focused on the types of phototherapies, such as NB-UVB and fractional CO2 laser. In addition, there is still some controversy regarding the efficacy of some drugs (such as TRL) in combination with EL for vitiligo (Citation17). Therefore, sorting, summarizing and analyzing the latest evidence on EL combination therapies for vitiligo will not only help to resolve the current controversy and provide some references for clinicians’ treatments, but also help to improve the guidelines for the diagnosis and treatment of adult vitiligo.
Limitations and advantages
The NMA has some limitations. First, the 11 studies included were all single-center studies. A lack of multicenter studies may have contributed to unstable results of the NMA. Second, some of the included studies had a small number of references on interventions and small sample sizes, which may have had an impact on the stability and feasibility of the conclusion. Third, the relative limitations of the data we extracted prevented us from performing a subgroup analysis, which may have had an impact on our final results. Fourth, although the studies included in the NMA were all RCTs, some of them were not investigator- or subject-blinded studies, and may have some bias. Fifth, since the number of subjects in some of the studies was 0, the data became e when we performed the NMA. We tried to process the data i.e., add 1 at the same time, but still existed when the processed data was analyzed. This has had some impact on our analysis to some extent. Finally, because the follow-up time in all the studies included in the NMA was 3–6 months, it was not possible to investigate the relationship between the follow-up time and the repigmentation rate and the long-term efficacy of different interventions. To address the above limitations, it is recommended to conduct large, high-quality, multicenter RCTs in future, so as to enhance blinding, add more important outcome measures, report the details on adverse events, and follow up subjects for a longer period of time.
Despite its limitations above, the NMA has some key advantages. First, all of the studies we included were RCTs, which is beneficial in reducing the risk of bias and ensuring validity and standardization of findings. Second, we included several newly published RCTs and evaluated the efficacy of EL combined AO for the first time, and also explored the advances in related research. Third, by using the NMA, we were able to combine data from the RCTs and simultaneously compare 9 interventions, including EL alone, EL combined with PRP, EL combined with CAL, and EL combined with CLO, which is a difficult problem for RCTs to solve. Fourth, we used the SUCRA values to rank the interventions for each outcome for simple visualization of which was more effective for each outcome by SUCRA values and SUCRA probability line charts.
Conclusion
Our NMA found that EL combined with AO was the preferred regimen for vitiligo, and EL alone was the regimen with the highest rate of treatment failure in vitiligo. Due to limited sample sizes of the included studies, larger, high-quality, multicenter RCTs are required to validate or update our findings.
Author contributions
ChanXiu Li: design, data collection and analysis, mapping, drafting; Yue Hu: design, data collection, mapping; ZengYi Mu: design, data collection, mapping; Lei Shi: design, data analysis, content modification; Xiao Sun: design, data collection, content modification; XinYue Wang: design, data collection, data interpretation; YaPing Wang: design, data collection; XinHong Li: design, manuscript revision, data interpretation, final approval.
Supplemental Material
Download PDF (199.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Diotallevi F, Gioacchini H, De Simoni E, et al. Vitiligo, from pathogenesis to therapeutic advances: state of the art. Int J Mol Sci. 2023;24(5):1. doi: 10.3390/ijms24054910.
- Alomrani AH, Alhazza FI, AlGhamdi KM, et al. Effect of neat and binary vehicle systems on the solubility and cutaneous delivery of piperine. Saudi Pharm J. 2018;26(2):162–12. doi: 10.1016/j.jsps.2017.12.015.
- Hu SH, Jiang S, Miao F, et al. sPmel17 secreted by ultraviolet B-Exposed melanocytes alters the intercellular adhesion of keratinocytes. Oxid Med Cell Longev. 2022;2022:1856830–1856812. doi: 10.1155/2022/1856830.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158(1):43–50. doi: 10.1001/jamadermatol.2021.4724.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36(10):1831–1844. doi: 10.1111/jdv.18257.
- Yang R, Zheng Y, Li L, et al. Direct conversion of mouse and human fibroblasts to functional melanocytes by defined factors. Nat Commun. 2014;5(1):5807. doi: 10.1038/ncomms6807.
- Picardo M, Huggins RH, Jones H, et al. The humanistic burden of vitiligo: a systematic literature review of quality-of-life outcomes. J Eur Acad Dermatol Venereol. 2022;36(9):1507–1523. doi: 10.1111/jdv.18129.
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451–459. doi: 10.2147/ccid.S185798.
- Lopes C, Trevisani VF, Melnik T. Efficacy and safety of 308-nm monochromatic excimer lamp versus other phototherapy devices for vitiligo: a systematic review with meta-analysis. Am J Clin Dermatol. 2016;17(1):23–32. doi: 10.1007/s40257-015-0164-2.
- Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38(1):621–648. doi: 10.1146/annurev-immunol-100919-023531.
- Sapam R, Agrawal S, Dhali TK. Systemic PUVA vs. narrowband UVB in the treatment of vitiligo: a randomized controlled study. Int J Dermatol. 2012;51(9):1107–1115. doi: 10.1111/j.1365-4632.2011.05454.x.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. Current and emerging treatments for vitiligo. J Am Acad Dermatol. 2017;77(1):17–29. doi: 10.1016/j.jaad.2016.11.010.
- Mohammad TF, Al-Jamal M, Hamzavi IH, et al. The vitiligo working group recommendations for narrowband ultraviolet B light phototherapy treatment of vitiligo. J Am Acad Dermatol. 2017;76(5):879–888. doi: 10.1016/j.jaad.2016.12.041.
- Bae JM, Jung HM, Hong BY, et al. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(7):666–674. doi: 10.1001/jamadermatol.2017.0002.
- Zhu B, Liu C, Zhang L, et al. Comparison of NB-UVB combination therapy regimens for vitiligo: a systematic review and network meta-analysis. J Cosmet Dermatol. 2023;22(3):1083–1098. doi: 10.1111/jocd.15534.
- Park KK, Liao W, Murase JE. A review of monochromatic excimer light in vitiligo. Br J Dermatol. 2012;167(3):468–478. doi: 10.1111/j.1365-2133.2012.11008.x.
- Bae JM, Hong BY, Lee JH, et al. The efficacy of 308-nm excimer laser/light (EL) and topical agent combination therapy versus EL monotherapy for vitiligo: a systematic review and meta-analysis of randomized controlled trials (RCTs). J Am Acad Dermatol. 2016;74(5):907–915. doi: 10.1016/j.jaad.2015.11.044.
- Bapur Erduran F, Adışen E. Comparison of the efficacy of 308-nm excimer lamp monotherapy with topical tacrolimus or clobetasol 17-propionate combination therapies in localized vitiligo. Photodermatol Photoimmunol Photomed. 2016;32(5–6):247–253. doi: 10.1111/phpp.12266.
- Nisticò S, Chiricozzi A, Saraceno R, et al. Vitiligo treatment with monochromatic excimer light and tacrolimus: results of an open randomized controlled study. Photomed Laser Surg. 2012;30(1):26–30. doi: 10.1089/pho.2011.3029.
- Soliman M, Samy NA, Abo Eittah M, et al. Comparative study between excimer light and topical antioxidant versus excimer light alone for treatment of vitiligo. J Cosmet Laser Ther. 2016;18(1):7–11. doi: 10.3109/14764172.2015.1052510.
- Juntongjin P, Toncharoenphong N. Effectiveness of a combined 308-nm excimer lamp and topical mid-potent steroid treatment for facial vitiligo: a preliminary, randomized double-blinded controlled study. Lasers Med Sci. 2020;35(9):2023–2029. doi: 10.1007/s10103-020-03048-5.
- Fenniche S, Zaouak A, Tanfous AB, et al. Successful treatment of refractory vitiligo with a combination of khellin and 308-nm excimer lamp: an open-label, 1-year prospective study. Dermatol Ther (Heidelb). 2018;8(1):127–135. doi: 10.1007/s13555-017-0218-x.
- Deng Y, Li J, Yang G. 308-nm excimer laser plus platelet-rich plasma for treatment of stable vitiligo: a prospective, randomized case-control study. Clin Cosmet Investig Dermatol. 2020;13:461–467. doi: 10.2147/ccid.S260434.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28(12):1610–1622. doi: 10.1111/jdv.12619.
- Farag HM, Yunusa I, Goswami H, et al. Comparison of amitriptyline and US food and drug administration-approved treatments for fibromyalgia: a systematic review and network meta-analysis. JAMA Netw Open. 2022;5(5):e2212939. doi: 10.1001/jamanetworkopen.2022.12939.
- Iheozor-Ejiofor Z, Gordon M, Clegg A, et al. Interventions for maintenance of surgically induced remission in crohn’s disease: a network meta-analysis. Cochrane Database Syst Rev. 2019;9(9):Cd013210. doi: 10.1002/14651858.CD013210.pub2.
- Loy JH, Merry SN, Hetrick SE, et al. Atypical antipsychotics for disruptive behaviour disorders in children and youths. Cochrane Database Syst Rev. 2017;8(8):Cd008559. doi: 10.1002/14651858.CD008559.pub3.
- Kanie T, Mizuno A, Takaoka Y, et al. Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;10(10):Cd013650. doi: 10.1002/14651858.CD013650.pub2.
- Elsaadany AE, El-Khalawany M, Elshahid AR, et al. Comparison between 308-nm excimer light alone versus 308-nm excimer light and platelet-rich plasma in the treatment for localized vitiligo. J Cosmet Dermatol. 2022;21(7):2826–2831. doi: 10.1111/jocd.14582.
- Khattab FM, Abdelbary E, Fawzi M. Evaluation of combined excimer laser and platelet-rich plasma for the treatment of nonsegmental vitiligo: a prospective comparative study. J Cosmet Dermatol. 2020;19(4):869–877. doi: 10.1111/jocd.13103.
- Juntongjin P, Sangganjanavanich P. Efficacy of the combined excimer light and topical calcipotriol for acral vitiligo: a randomized double-blind comparative study. Dermatol Ther. 2021;34(2):e14886. doi: 10.1111/dth.14886.
- Lu-Yan T, Wen-Wen F, Lei-Hong X, et al. Topical tacalcitol and 308-nm monochromatic excimer light: a synergistic combination for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2006;22(6):310–314. doi: 10.1111/j.1600-0781.2006.00250.x.
- Ghalamkarpour F, Robati R, Ghasir G, et al. The 308-nm xenon chloride excimer laser in combination with topical calcipotriol in the treatment of vitiligo. Iran J Dermatol. 2011;14(1):12–15.
- Sassi F, Cazzaniga S, Tessari G, et al. Randomized controlled trial comparing the effectiveness of 308-nm excimer laser alone or in combination with topical hydrocortisone 17-butyrate cream in the treatment of vitiligo of the face and neck. Br J Dermatol. 2008;159(5):1186–1191. doi: 10.1111/j.1365-2133.2008.08793.x.
- Passeron T, Ostovari N, Zakaria W, et al. Topical tacrolimus and the 308-nm excimer laser: a synergistic combination for the treatment of vitiligo. Arch Dermatol. 2004;140(9):1065–1069. doi: 10.1001/archderm.140.9.1065.
- Kawalek AZ, Spencer JM, Phelps RG. Combined excimer laser and topical tacrolimus for the treatment of vitiligo: a pilot study. Dermatol Surg. 2004;30(2 Pt 1):130–135. doi: 10.1111/j.1524-4725.2004.30058.x.
- Chen J, Li S, Li C. Mechanisms of melanocyte death in vitiligo. Med Res Rev. 2021;41(2):1138–1166. doi: 10.1002/med.21754.
- Sun C, Shan F, Liu M, et al. High-fat-diet-induced oxidative stress in giant freshwater prawn (macrobrachium rosenbergii) via NF-κB/NO signal pathway and the amelioration of vitamin E. Antioxidants (Basel). 2022;11(2):228. doi: 10.3390/antiox11020228.
- Ma J, Li S, Zhu L, et al. Baicalein protects human vitiligo melanocytes from oxidative stress through activation of NF-E2-related factor2 (Nrf2) signaling pathway. Free Radic Biol Med. 2018;129:492–503. doi: 10.1016/j.freeradbiomed.2018.10.421.
- Arowojolu OA, Orlow SJ, Elbuluk N, et al. The nuclear factor (erythroid-derived 2)-like 2 (NRF2) antioxidant response promotes melanocyte viability and reduces toxicity of the vitiligo-inducing phenol monobenzone. Exp Dermatol. 2017;26(7):637–644. doi: 10.1111/exd.13350.
- Jian Z, Li K, Song P, et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. J Invest Dermatol. 2014;134(8):2221–2230. doi: 10.1038/jid.2014.152.
- Zedan H, Abdel-Motaleb AA, Kassem NM, et al. Low glutathione peroxidase activity levels in patients with vitiligo. J Cutan Med Surg. 2015;19(2):144–148. doi: 10.2310/7750.2014.14076.
- Kang P, Chen J, Zhang W, et al. Oxeiptosis: a novel pathway of melanocytes death in response to oxidative stress in vitiligo. Cell Death Discov. 2022;8(1):70. doi: 10.1038/s41420-022-00863-3.
- Watanabe S, Kawamoto S, Ohtani N, et al. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108(4):563–569. doi: 10.1111/cas.13184.
- Lei T-C, Xu A-E, Gao T-W, et al. Consensus on the diagnosis and treatment of vitiligo in China (2021 revision). Int J Dermatol Venereol. 2021;4(1):10–15. doi: 10.1097/JD9.0000000000000151.
- Dellatorre G, Antelo DAP, Bedrikow RB, et al. Consensus on the treatment of vitiligo - Brazilian society of dermatology. An Bras Dermatol. 2020;95(Suppl 1):70–82. doi: 10.1016/j.abd.2020.05.007.
- Taieb A, Alomar A, Böhm M, et al. Guidelines for the management of vitiligo: the european dermatology forum consensus. Br J Dermatol. 2013;168(1):5–19. doi: 10.1111/j.1365-2133.2012.11197.x.
- Leone G, Paro Vidolin A. Effect of an antioxydant cream versus placebo in patients with vitiligo in association with excimer laser. A pilot randomized, investigator-blinded, and half-side comparison trial. G Ital Dermatol Venereol. 2015;150(4):461–466.
- Chang WL, Ko CH. The role of oxidative stress in vitiligo: an update on its pathogenesis and therapeutic implications. Cells. 2023;12(6):936. doi: 10.3390/cells12060936.
- Zhang S, Yi X, Su X, et al. Ginkgo biloba extract protects human melanocytes from H(2) O(2)-induced oxidative stress by activating Nrf2. J Cell Mol Med. 2019;23(8):5193–5199. doi: 10.1111/jcmm.14393.
- Zhou Y, Khan M, Jiang L, et al. The current status of antioxidants in the treatment of vitiligo in China. Oxid Med Cell Longev. 2022;2022:2994558–2994513. doi: 10.1155/2022/2994558.
- Silpa-Archa N, Lim HW, Wongpraparut C. Excimer laser in vitiligo: where there is light, there is hope. Br J Dermatol. 2019;181(1):21–22. doi: 10.1111/bjd.18101.
- Bae JM, Kwon SH, Ju HJ, et al. Suberythemic and erythemic doses of a 308-nm excimer laser treatment of stable vitiligo in combination with topical tacrolimus: a randomized controlled trial. J Am Acad Dermatol. 2020;83(5):1463–1464. doi: 10.1016/j.jaad.2020.03.009.
- Post NF, Ezekwe N, Narayan VS, et al. The use of lasers in vitiligo, an overview. J Eur Acad Dermatol Venereol. 2022;36(6):779–789. doi: 10.1111/jdv.18005.