Abstract
Background
Rituximab (RTX) is considered the first-line treatment for pemphigus vulgaris (PV), which is a B-cell-mediated acquired autoimmune disease. However, no consensus on the optimum dosage has been achieved.
Objectives
To investigate the efficacy and safety of low-dose RTX (a single infusion of 500 mg) for the treatment of PV, a cohort study was conducted for patients with PV, along with a 12-month follow-up following the administration of RTX.
Methods
Patients with moderate or severe PV were divided into group A (low-dose RTX combined with corticosteroids) and group B (corticosteroids alone). Data on complete remission (CR) rates, doses of corticosteroids, cumulative doses of corticosteroids at the third, sixth, and twelfth months, pemphigus disease area index and adverse effects (AEs) were collected.
Results
Forty-four patients with moderate or severe PV were enrolled in this study (19 in group A and 25 in group B). Patients treated with low-dose RTX had higher CR rates, lower doses of corticosteroids at the third, sixth, and twelfth months, lower cumulative doses of corticosteroids at the sixth and twelfth months, and fewer AEs than those who received corticosteroids alone.
Conclusions
This study indicated that low-dose RTX may be a beneficial and secure therapy option for patients with moderate to severe PV.
Introduction
Pemphigus vulgaris (PV) is a B-cell-mediated acquired autoimmune disease and is characterized by erythema, loose blisters, and erosion of the mucosa and skin (Citation1). The pathogenesis of PV is characterized by the formation of autoantibodies directed against desmoglein (Dsg) 1 and 3, which could disrupt the intraepidermal adhesion and lead to blister formation (Citation2,Citation3). The first-line therapy for PV is systemic corticosteroids, either alone or in combination with immunosuppressants. Mortality attributed to PV has decreased from 75% to 5–10% of patients who received these treatments (Citation4–6). With the introduction of targeted therapies, rituximab (RTX), a chimeric murine/human monoclonal antibody that binds to the CD-20 antigen expressed on B-cells, has demonstrated efficacy in the treatment of PV by depleting the number of B-cells (Citation7–10). Treatment regimens that utilize RTX regimens for patients with PV increase the chance of the patient achieving complete remission (CR) to almost three times higher than the corticosteroids-alone regimen (Citation11,Citation12). A lymphoma protocol (375 mg/m2 each week × 4 times) in 2002 was the first to use RTX to treat refractory PV (Citation13). In addition to this protocol, the rheumatoid arthritis (RA) protocol (1000 mg at 2 week intervals × 2 times) is also recommended by the international expert consensus as the first-line treatment for PV (Citation14). When administered at a dosage of less than 1 mg/m2, RTX can deplete circulating B-cells in healthy individuals (Citation15,Citation16). Additionally, because of factors such as the expense of the full-dosage medication and the risk of infection caused by depletion of B-cells and infusion reactions, the clinical utility of the full dose of the drug is often limited. Various studies have investigated different RTX dosages for the treatment of PV (Citation17–20), for example, Kim et al. found that time to achieve CR and relapse rates were significantly lower in the patients trearted with three to more infusions of RTX (each 375 mg/m2) than that of two infusions of RTX (Citation19). However, in another randomized controlled trial performed by Kanwar et al. (Citation21), they found no statistically significant difference between groups (2 infusions of 1000 mg vs. 2 infusions of 500 mg) in terms of time to disease control and CR rates. Therefore, no consensus has been reached on the optimal dosage regimen so far. In addition, most previous studies have focused on the efficacy of two to more infusions of RTX (each 500 mg or 375 mg/m2) in the treatment of PV (Citation21–23), but few studies have investigated RTX (≤ 500 mg for a cycle) in the treatment of PV (Citation24). The purpose of this study was to compare the efficacy and safety of low-dose RTX (a single infusion of 500 mg) combined with corticosteroids versus corticosteroids alone in the treatment of patients with moderate to severe PV in China. We hope this study could provide a foundation and point of reference for further research toward a more suitable and cost-effective protocol for treating PV with RTX.
Materials and methods
Study sites
This single-center, non-randomized open-label cohort study was conducted at the Department of Dermatology, West China Hospital (WCH), Sichuan University, China, between 31 December 2018, and 31 April 2021.
Study participants and enrollment criteria
All participants were diagnosed with moderate or severe PV at WCH during the study period and were recruited after they signed an informed consent. The inclusion criteria were as follows: (i) aged 18–70 years, (ii) confirmed moderate or severe PV. Diagnosis required clinical presentation (flaccid blisters and erosions involving the skin and/or oral mucosa), PV-consistent histopathology (epidermal acantholysis), and either positive direct immunofluorescence (IgG and/or complement component 3 deposits observed in the stratified squamous epithelium) or serological detection of IgG antibodies against Dsg 1 and/or Dsg3 by enzyme-linked immunosorbent assay (ELISA). Disease severity was based on established grading criteria and used the pemphigus disease area index (PDAI; 0–8 for mild, 9–24 for moderate, and ≥25 for severe disease) (Citation25). Exclusion criteria: (i) those working in disease control who had been treated with corticosteroids, immunosuppressants, human immunoglobulin, or biological agents before inclusion; (ii) those with active or chronic infections, including active or latent tuberculosis, active or chronic hepatitis, immunodeficiency disorders, malignant tumors, or organs dysfunction including hepatopathy, chronic renal failure, cardiovascular/pulmonary diseases, and neurologic/psychiatric disorders; (iii) women who were pregnant or had plans to get pregnant; (iv) those who were reluctant to provide informed consent or were unable to complete the entire study.
Treatment protocols and outcome measures
Patients were nonrandomly divided into group A (a single infusion of 500 mg RTX combined with corticosteroids) and group B (corticosteroids alone) after being enrolled. The dose reduction of prednisone was performed according to the clinical evaluation (PDAI) of disease activity. After the initiation of therapy, the dose of prednisone was reduced when the patient’s disease activity was controlled (new lesions cease to form and established lesions begin to heal). In the corticosteroids alone group, the dose of prednisone was reduced by 5 mg every 2 weeks when the dose of prednisone was more than 40 mg. Then the dose of prednisone was reduced by 5 mg every month when the dose of prednisone was between 20–40 mg, and following reduction of 2.5 mg every 3 months when the dose of prednisone was less than 20 mg. For the patients receiving RTX in combination with corticosteroids, the dose of prednisone was reduced by 5 mg every 2 weeks when the dose of prednisone was more than 40 mg. Then we reduced the dose of prednisone by 10 mg every month when the dose of prednisone was between 20–40 mg, and following reduction of 5 mg every 3 months when the dose of prednisone was less than 20 mg. The observation endpoint of this study was 12 months. Information about baseline characteristics, medication status, complete remission (CR) rates (CR in this study maeas CR during therapy: the absence of new or established lesions while the patient is receiving minimal therapy; minimal therapy: prednisolone (or the equivalent) at a dose of 10 mg/d) and time of CR, individual and cumulative doses of corticosteroids at the third, sixth, and twelfth months, B-cell counts, PDAI, and adverse effects (AEs) was collected.
Statistical analysis
The quantitative data were summarized by mean (standard deviation, SD) or median (interquartile range, IQR), and the qualitative data were summarized by ratio or proportion. A t-test was used to compare the baseline characteristics across two groups for quantitative data with normal distribution, a Wilcoxon rank sum test was used for data without normal distribution, and Fisher’s exact test was employed for dichotomous responses. Values were considered significant when p < 0.05. The occurrence of CR was set as the outcome of survival analysis (with CR = 1, without CR = 0). A Kaplan-Meier survival curve was created to illustrate the survival time of each group, and a log-rank test was used to compare them. The survival time was modeled as a function of gender, age, initial PDAI score, disease activity, involvement of skin or mucosa, anti-Dsg1/3 antibodies, initial doses of prednisone, and dosing regimen. A Cox regression model was subsequently employed to determine which regimen led to higher CR rates.
Results
Patient characteristics
Overall, 44 eligible patients with moderate or severe PV were included in our study. Patients were divided into two groups: 19 patients were in group A and 25 were in group B. Demographic characteristics (sex, age and weight), disease activity (PDAI), involvement of skin and mucosa, and positivity of anti-Dsg1/3 antibodies did not significantly differ between the two groups ().
Table 1. Comparison of baseline characteristics between two groups.
Comparison of outcome indicators between two groups
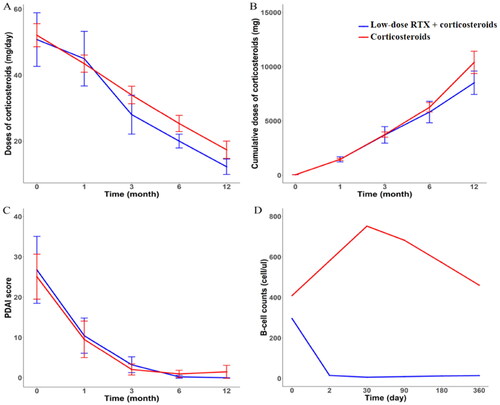
The CR rates of patients in group A were significantly higher than those in group B. The initial doses of corticosteroids (mg prednisone equivalent) were the same between the two groups. The doses of corticosteroids in group A at the third, sixth, and twelfth months and the cumulative doses of corticosteroids at the sixth and twelfth months were significantly lower than those in group B (). These results indicated that RTX combined with corticosteroids could increase the CR rates of patients with PV, accelerate the tapering of corticosteroids, and reduce the cumulative doses of corticosteroids.
Table 2. Comparison of outcome indicators between two groups.
Changes in doses of corticosteroids and pemphigus disease area index between the two groups
All patients showed a steady reduction from baseline in daily corticosteroid dose, but the change was not statistically different between the two groups (). Although without statistical significance, the upward trend of cumulative doses of corticosteroids in group A was slower than that of group B, (). No statistical difference was found between the two groups in the reduction of PDAI score during the 12-month follow-up period. However, the PDAI score of group B increased slightly after six months, which suggested that the disease activity of group B may have fluctuated ().
Changes in B-cell counts between two groups
B-cell counts of patients in group A decreased after the second day of treatment, then continued to decrease at follow-up visits and remained at less than 10 cells/ul at the first and third month follow-ups. Although they had increased slightly by the sixth and the twelfth month follow-ups, they were still always below the inferior limit (< 15 cells/ul), and they were also lower than those of the patients in group B. The B-cell counts of patients in group B increased at the beginning of treatment, then decreased during the follow-up period, but they were still always higher than the normal range ().
Survival analysis between two groups
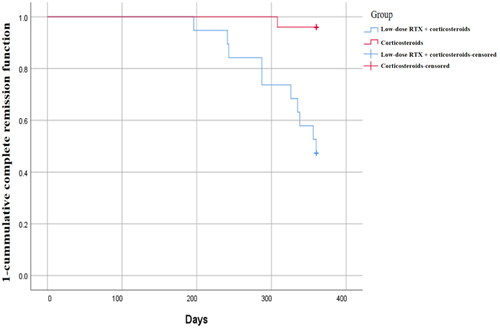
A Kaplan-Meier survival curve showed that, by the endpoint of the study, more patients achieved CR in group A than in group B. In addition, the 1-cumulative CR function of group A was always lower than that of group B, which may imply that in terms of CR indicator, treatment for group A was consistently more effective than the treatment for group B at any observation point. A statistical difference in the cumulative CR functions between the two groups was found by the Log-rank test (Chi-square = 13.966, p < 0.001) (). A Cox analysis () showed the dosage regimen was a factor influencing complete remission status (HR = 41.112, p < 0.01), and that the dosage regimen of group A was more effective than that of group B after controlling for other confounding factors.
Table 3. COX Proportional hazard regression analysis between two groups.
Adverse effects between two groups
Twelve AEs were reported in 10 patients in group A, and 33 AEs were reported in 14 patients in group B (). Elevation of liver enzyme occurred most frequently in group A (three events, 25%). Other AEs included folliculitis, metabolic abnormalities (hyperlipidemia, hypokalemia, diabetes mellitus), hypertension, and viral infection, all of which were mild. The most common AEs in group B were bone abnormality including osteoporosis, pathological fracture, osteonecrosis (11 events, 33%). Other AEs in group B included hypothyroidism (one event, 3%), occipital nerve injury (one event, 3%), and steroid acne (one event, 3%). In group B, nine serious AEs (27.3%) were reported: pulmonary bacterial infection (three events, 9%), encephalopyosis caused by Nocardia (one event, 3%), pathologic fracture (three events, 9%), osteonecrosis (one event, 3%), and stress ulcer with bleeding (one event, 3%). Fewer AEs, especially severe AEs, occurred in patients treated with ultra-low-dose RTX combined with corticosteroids than in those who were treated with corticosteroids alone.
Table 4. Adverse events between two groups.
Discussion
Low-dose RTX has been reported to be effective in treating PV, but research was limited to only a few cases or case series (Citation24,Citation26). To our knowledge, this was the first cohort study to compare the efficacy and safety of low-dose RTX (a single infusion of 500 mg) combined with corticosteroids and corticosteroids alone for the treatment of PV. Ten of 19 patients (52.63%) in the RTX group achieved CR, which was much higher than the rate in those treated with corticosteroids alone (one of 25 patients, 4.00%). The CR rates reported from previous studies of RTX for PV treatment range from 70%-100% on a high-dose regimen and 35%-100% on other low-dose regimens (Citation7,Citation15,Citation27,Citation28). These differences may be explained by the different endpoints and variable dosages of corticosteroids after RTX administration.
In our study, Time to CR was 243 days in the low-dose RTX group and 294 days in the corticosteroids alone group. Comparison cannot be employed to this result due to the large sampling variance raised by only one patient achieving CR in the corticosteroids alone group. However, considering that PV is a rare disease and this was a single-center study; although we couldn’t perform a comparison, we still report the CR time of this only one patient in the corticosteroids alone group as a case study, which may provide implications for future studies. Time to CR for PV patients treated with low-dose RTX reported by other researchers fluctuated from 106 to 433 days (Citation19,Citation23,Citation29,Citation30), and for patients treated with high-dose RTX was usually no longer than 200 days (Citation19,Citation28,Citation29). A meta-analysis involving 30 studies and 578 patients reported that, compared with low-dose RTX, high-dose RTX was not associated with statistically significant time to CR (Citation31). Comparison on whether using our regimen to achieve CR is more rapid than low-dose or high-dose regimen was limited by the variations of study designs (for example, inclusion and exclusion criteria, follow-up time) and alternative outcome indicators. Except for the time to CR, during the 12-month follow-up, all patients in both groups showed a steady reduction from baseline in daily corticosteroid dosage. The RTX combined with corticosteroids helped to rapidly reduce the corticosteroids, and also reduced the cumulative doses of corticosteroids.
Rapid depletion of circulating B-cells was observed after RTX administration (Citation32). In our study, B-cell counts decreased rapidly on the second day after using RTX and were maintained at very low levels for 12 months. The repopulation of B-cells in peripheral blood usually occurs within 6–9 months of one course of treatment with RTX (Citation33), so the re-treatment with RTX was usually performed after six months (Citation34). Nevertheless, some reports showed patients may achieve long-lasting remission even with a single infusion of RTX (Citation34,Citation35). Schoergenhofer et al. reported that two infusions of 100 mg of RTX may suffice to fully suppress B-cells for three months (Citation16). Additional prophylactic infusion of RTX may affect the reconstitution of immunity for several months afterward, which increases the risk of AEs. Some researchers suggest identifying individual factors and eventual biomarkers to determine which patients have a higher risk of relapse, and giving them a subsequent infusion of RTX (Citation24). No patient in our study relapsed within 12 months of one cycle of 500 mg RTX. The results suggest that low-dose RTX for PV may effectively eliminate B-cells as well as maintain a long duration of B-cell depletion. We also propose that the second dose of RTX could be used until the repopulation of B-cells or relapse of patients, which would be also cost-effective for patients with PV.
Usually, RTX is well-tolerated with few life-threatening AEs. Most patients have mild AEs, such as fever, cold, nausea, hypotension, thrombocytopenia and rash, and these symptoms usually decrease or disappear after infusion (Citation36,Citation37). In our study, three patients (15.8%) in the low-dose RTX group experienced AEs of minor infections. Among patients treated with corticosteroids alone, 8 patients had nine serious AEs, including osteonecrosis, pathological fracture, stress ulcer with bleeding, and encephalopyosis (Nocardia). These serious AEs could be disabling or even life-threatening to patients with PV. No serious AEs occurred in our low-dose RTX group, which may indicate that the low-dose of RTX combined with corticosteroids was safer than corticosteroids alone for the treatment of patients with moderate to severe PV.
In conclusion, this study showed that low-dose RTX may be sufficient, safe and cost-effective to control the disease activity for patients with moderate to severe PV. Maybe the repeated infusions could be performed based on the patient’s clinical and laboratory condition in the follow-up period. Meanwhile, our study was limited due to its small sample size, and only 12 months of follow-up period. Prospective randomized controlled trials with longer follow-up periods should be performed to investigate these findings further.
Ethical approval
The study protocol was approved by the Ethics Committee of WCH, Sichuan University (2017, No. 96) and registered in the Chinese Clinical Trial Registry (ChiCTR 1800020382). The patients provided their written informed consent to participate in this study.
Author contributions
Wei Li contributed to the conception of the study and made constructive discussions in manuscript; Xingli Zhou, Tongying Zhan, Xiaoxi Xu mainly helped perform the study and wrote the manuscript; Tianjiao Lan performed the data analysis; Jinqiu Wang and Dengmei Xia helped perform the evaluations of PDAI and record the occurrence of AEs during the follow-up period; Hongxiang Hu, Yiyi Wang, Yue Xiao and Yuxi Zhou contributed to the collection of laboratory results and data recording.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394(10201):1–7. doi:10.1016/s0140-6736(19)31778-7.
- Lanza A, Cirillo N, Femiano F, et al. How does acantholysis occur in pemphigus vulgaris: a critical review. J Cutan Pathol. 2006;33(6):401–412. doi:10.1111/j.0303-6987.2006.00523.x.
- Melchionda V, Harman KE. Pemphigus vulgaris and pemphigus foliaceus: an overview of the clinical presentation, investigations and management. Clin Exp Dermatol. 2019;44(7):740–746. doi:10.1111/ced.14041.
- Bystryn JC, Steinman NM. The adjuvant therapy of pemphigus. Arch Dermatol. 1996;132(2):203–212. doi:10.1001/archderm.1996.03890260105016.
- Martin LK, Werth VP, Villaneuva EV, et al. A systematic review of randomized controlled trials for pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. 2011;64(5):903–908. doi:10.1016/j.jaad.2010.04.039.
- Lever WF, White H. Treatment of pemphigus with corticosteroids. Results obtained in 46 patients over a period of 11 years. Arch Dermatol. 1963;87(1):12–26. doi:10.1001/archderm.1963.01590130018006.
- Tavakolpour S, Aryanian Z, Seirafianpour F, et al. A systematic review on efficacy, safety, and treatment-durability of low-dose rituximab for the treatment of pemphigus: special focus on COVID-19 pandemic concerns. Immunopharmacol Immunotoxicol. 2021;43(5):507–518. doi:10.1080/08923973.2021.1953063.
- Zambruno G, Borradori L. Rituximab immunotherapy in pemphigus: therapeutic effects beyond B-cell depletion. J Invest Dermatol. 2008;128(12):2745–2747. doi:10.1038/jid.2008.330.
- Hertl M, Jedlickova H, Karpati S, et al. Pemphigus. S2 guideline for diagnosis and treatment–guided by the European dermatology forum (EDF) in cooperation with the European academy of dermatology and venereology (EADV). J Eur Acad Dermatol Venereol. 2015;29(3):405–414. doi:10.1111/jdv.12772.
- Musette P, Bouaziz JD. B cell modulation strategies in autoimmune diseases: new concepts. Front Immunol. 2018;9:622. doi:10.3389/fimmu.2018.00622.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031–2040. doi:10.1016/s0140-6736(17)30070-3.
- Alaeen H, Toosi R, Mahmoudi H, et al. Short-term clinical and serological follow-up with conventional and conformational anti-desmoglein antibodies in treatment-naïve and previously treated patients with pemphigus vulgaris after receiving rituximab. Int J Womens Dermatol. 2019;5(5):372–377. doi:10.1016/j.ijwd.2019.05.008.
- Salopek TG, Logsetty S, Tredget EE. Anti-CD20 chimeric monoclonal antibody (rituximab) for the treatment of recalcitrant, life-threatening pemphigus vulgaris with implications in the pathogenesis of the disorder. J Am Acad Dermatol. 2002;47(5):785–788. doi:10.1067/mjd.2002.126273.
- Murrell DF, Peña S, Joly P, et al. Diagnosis and management of pemphigus: recommendations of an international panel of experts. J Am Acad Dermatol. 2020;82(3):575–585 e571. doi:10.1016/j.jaad.2018.02.021.
- Thukral S, Shinde N, Ray DS. Effect of different rituximab doses on B cell count, anti-a/B antibody titer, graft function, and infectious complications in ABO-Incompatible renal transplantation: a prospective study. Transplant Proc. 2021;53(3):970–975. doi:10.1016/j.transproceed.2020.10.008.
- Schoergenhofer C, Schwameis M, Firbas C, et al. Single, very low rituximab doses in healthy volunteers - a pilot and a randomized trial: implications for dosing and biosimilarity testing. Sci Rep. 2018;8(1):124. doi:10.1038/s41598-017-17934-6.
- Yancey KB. Commentary regarding clinical and immunological outcomes of high- and low-dose rituximab treatments in patients with pemphigus: a randomized, comparative, observer-blinded study. Dermatol Ther. 2015;28(3):174–175. doi:10.1111/dth.12198.
- Alaibac M. Ultra-Low dosage regimen of rituximab in autoimmune blistering skin conditions. Front Immunol. 2018;9:810. doi:10.3389/fimmu.2018.00810.
- Kim JH, Kim YH, Kim MR, et al. Clinical efficacy of different doses of rituximab in the treatment of pemphigus: a retrospective study of 27 patients. Br J Dermatol. 2011;165(3):646–651. doi:10.1111/j.1365-2133.2011.10411.x.
- Londhe PJ, Kalyanpad Y, Khopkar US. Intermediate doses of rituximab used as adjuvant therapy in refractory pemphigus. Indian J Dermatol Venereol Leprol. 2014;80(4):300–305. doi:10.4103/0378-6323.136832.
- Kanwar AJ, Vinay K, Sawatkar GU, et al. Clinical and immunological outcomes of high- and low-dose rituximab treatments in patients with pemphigus: a randomized, comparative, observer-blinded study. Br J Dermatol. 2014;170(6):1341–1349. doi:10.1111/bjd.12972.
- Gupta J, Raval RC, Shah AN, et al. Low-dose rituximab as an adjuvant therapy in pemphigus. Indian J Dermatol Venereol Leprol. 2017;83(3):317–325. doi:10.4103/ijdvl.IJDVL_1078_14.
- Horváth B, Huizinga J, Pas HH, et al. Low-dose rituximab is effective in pemphigus. Br J Dermatol. 2012;166(2):405–412. doi:10.1111/j.1365-2133.2011.10663.x.
- Russo I, Miotto S, Saponeri A, et al. Ultra-low dose rituximab for refractory pemghigus vulgaris: a pilot study. Expert Opin Biol Ther. 2020;20(6):673–678. doi:10.1080/14712598.2020.1727440.
- Shimizu T, Takebayashi T, Sato Y, et al. Grading criteria for disease severity by pemphigus disease area index. J Dermatol. 2014;41(11):969–973. doi:10.1111/1346-8138.12649.
- Simpson K, Low ZM, Yap T, et al. Ultralow-dose rituximab in pemphigus: a single-Centre experience. Br J Dermatol. 2022;186(3):581–583. doi:10.1111/bjd.20819.
- Chang Y, Chen X, Wang M, et al. A 10-year retrospective cohort analysis of rituximab for the treatment of pemphigus in a chinese population. J Am Acad Dermatol. 2021;85(6):1643–1645. doi:10.1016/j.jaad.2020.12.062.
- Zhang J, Huang X, Zhang Z, et al. Clinical observation of different doses of rituximab for the treatment of severe pemphigus: a single-center prospective cohort study. J Am Acad Dermatol. 2022;88(2):500–502. doi:10.1016/j.jaad.2022.06.1187.
- Cho HH, Jin SP, Chung JH. Clinical experiences of different dosing schedules of rituximab in pemphigus with various disease severities. J Eur Acad Dermatol Venereol. 2014;28(2):186–191. doi:10.1111/jdv.12080.
- Singh N, Handa S, Mahajan R, et al. Comparison of the efficacy and cost-effectiveness of an immunologically targeted low-dose rituximab protocol with the conventional rheumatoid arthritis protocol in severe pemphigus. Clin Exp Dermatol. 2022;47(8):1508–1516. doi:10.1111/ced.15213.
- Wang HH, Liu CW, Li YC, et al. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm Venereol. 2015;95(8):928–932. doi:10.2340/00015555-2116.
- Mohammed R, Milne A, Kayani K, et al. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J Blood Med. 2019;10:71–84. doi:10.2147/jbm.s190784.
- Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther. 2013;15 Suppl 1(Suppl 1):S3. doi:10.1186/ar3908.
- Didona D, Maglie R, Eming R, et al. Pemphigus: current and future therapeutic strategies. Front Immunol. 2019;10:1418. doi:10.3389/fimmu.2019.01418.
- Hebert V, Joly P. Rituximab in pemphigus. Immunotherapy. 2018;10(1):27–37. doi:10.2217/imt-2017-0104.
- Gopal AK, Press OW. Clinical applications of anti-CD20 antibodies. J Lab Clin Med. 1999;134(5):445–450. doi:10.1016/s0022-2143(99)90164-6.
- Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90(6):2188–2195. doi:10.1182/blood.V90.6.2188.