Abstract
Both bullous pemphigoid (BP) and psoriasis are common immune-related dermatological conditions in clinical practice, but the co-occurrence of these two diseases is rare. Currently, there is no consensus on the long-term safe and effective treatment for patients with both BP and psoriasis. JAK inhibitors are emerging as targeted therapeutic drugs that act by inhibiting Janus kinase activity, regulating the JAK/STAT pathway, blocking the transduction pathway of key proinflammatory cytokines, and influencing T-cell differentiation. These cytokines upstream of the JAK/STAT pathway play a pivotal role in the pathogenesis of numerous inflammatory and autoimmune disorders. Upadacitinib, a second-generation JAK inhibitor with high selectivity, demonstrates promising potential.
This case report aims to provide a description of the successful treatment of bullous pemphigoid (BP) and psoriasis vulgaris by using upadacitinib, highlighting significant clinical outcomes. Additionally, we aim to analyze the underlying mechanism of upadacitinib in treating these two comorbidities by reviewing relevant literature from both domestic and international sources. Based on our clinical observations, upadacitinib appears to be a promising and well-tolerated therapeutic option for patients with concurrent BP and psoriasis, offering valuable insights for developing appropriate treatment strategies in clinical practice.
1. Introduction
Bullous pemphigoid (BP) is an autoimmune blistering skin disease, characterized by severe pruritus and the formation of blisters beneath the epidermis. Its pathogenesis involves an imbalance in Th1/Th2 subsets, with a predominance of Th2 cytokines (Citation1). Psoriasis is an immune-mediated chronic, recurrent, inflammatory, and systemic disease, influenced by both genetic and environmental factors. The typical clinical manifestation includes localized or widespread scaly erythema or plaques, wherein dysfunctional helper T cells (Th1, Th17, etc.) play a crucial role in its pathogenesis (Citation2). Both bullous pemphigoid and psoriasis are immune-related dermatological conditions that have garnered increasing attention in recent years. The precise mechanisms underlying their pathogenesis remain unclear; however, it is speculated that they may involve immune mechanisms, inflammatory cells, cytokines among others. Given the conflicting treatment approaches for BP and psoriasis management strategies available currently, there exists significant importance in identifying effective therapeutic interventions.
The JAK/STAT signaling pathway is a widely expressed signal transduction pathway involved in various biological processes, including cell proliferation, apoptosis, and immune regulation. It plays a crucial role in the development of inflammatory diseases (Citation3,Citation4), autoimmune disorders, and malignant tumors by activating JAKs and STATs. Due to its potent regulatory effect, small molecule targeted drugs known as JAK inhibitors (JAKi) have emerged as a promising research focus for the treatment of immune-related skin diseases. JAKi can efficiently hinder JAK activation and obstruct the STAT pathway by attaching to the adenosine triphosphate (ATP) binding site on proteins, thus accomplishing therapeutic objectives (Citation5). Notably, first-generation JAK inhibitors such as baricitinib or tofacitinib have shown efficacy in treating mucosal pemphigoid (Citation6,Citation7), while different targets alone or in combination inhibition by JAK inhibitors has demonstrated significant therapeutic effects in psoriasis (Citation8). Furthermore, case reports have highlighted successful treatment outcomes using baricitinib or tofacitinib for patients with both BP and psoriasis (Citation9,Citation10). However, there remains no consensus regarding the optimal treatment strategy for utilizing JAKi in cases of BP complicated with psoriasis.
Upadacitinib, a highly selective JAK1 inhibitor, exhibits superior clinical potential compared to first-generation JAK inhibitors such as baricitinib and tofacitinib, due to its specific targeting of JAK1, resulting in a significant reduction in the incidence of adverse reactions while effectively managing disease progression. A case study demonstrated the successful treatment of BP with upadacitinib (Citation11), while another case report illustrating the successful use of upadacitinib in treating nail psoriasis (Citation12). Moreover, upadacitinib has obtained approval for the management of psoriatic arthritis. However, no studies have assessed the efficacy of upadacitinib in the combined treatment of BP and psoriasis.
This paper presents a case study of bullous pemphigoid in association with psoriasis vulgaris, wherein the patient was effectively managed using upadacitinib. Additionally, an extensive review of relevant literature from both domestic and international sources has been conducted to summarize the clinical experience of upadacitinib in treating the combined condition of bullous pemphigoid and psoriasis.
2. Case data
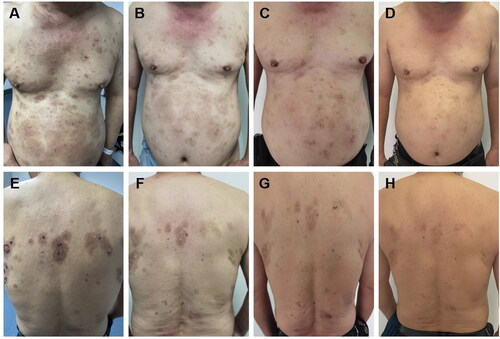
The patient, a 66-year-old man, presented with erythema, blisters, and pruritus on the trunk, persisting for over 2 months. Initially, abdominal erythema and papules accompanied by pruritus appeared without apparent triggers. Subsequently, the skin lesions gradually extended to involve the entire body, primarily manifesting as chest, abdomen, and back erythema, along with thick-walled blisters and bullae superimposed on the erythematous base, accompanied by pronounced itching. Erosion, exudation, and crusting ensued following scratching. The patient was admitted to the dermatology ward with a confirmed diagnosis of “bullous pemphigoid”. Past medical history revealed a more than 10-year-long history of controlled psoriasis without cutaneous manifestations. Upon admission, physical examination unveiled multiple trunk erythema and papules alongside ulcerated blisters on the chest and back, exhibiting partial erosion and crusting, but devoid of hemorrhagic or purulent discharge; scattered scratch marks were observed along with pigmentation, lesions covering over 40% of the body-surface area ().
Figure 1. A, E: Clinical features of bullous pemphigoid lesions on the skin of a patient upon initial diagnosis (lesions covering over 40% of the body-surface area). B, F: following a two-week therapy regimen involving the administration of Dupilumab in conjunction with low-dose hormones, a notable reduction in the presence of lesions was seen (lesions covering 35% of the body-surface area). C, G: following a 4-week therapy protocol using only upadacitinib, a significant improvement in the condition of the skin lesions was noted (lesions covering 10% of the body-surface area)., with no occurrence of new lesions. D, H: following a sustained 10-week course of upadacitinib treatment, the regression of pre-existing lesions persisted (lesions covering < 5% of the body-surface area), with no new lesions observed.
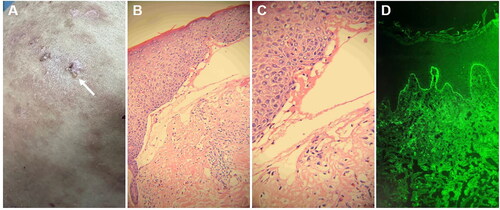
Pertinent laboratory investigations: The serological detection of antibodies against bullous skin (ELISA): anti-epidermal basement membrane antibody (+), anti-epidermal intercellular desmosome anti-body (−), anti-DSG-1 (−), anti-DSG-3 (−), anti-BP180 (±), anti-BP230 (−). A blister that remained intact on the abdomen was collected for skin pathological analysis (). The skin pathology revealed the presence of clefts beneath the epithelial basement membrane, accompanied by exudation of numerous acute and chronic inflammatory cells within these clefts. Additionally, there was infiltration of lymphocytes, plasma cells, and eosinophils in the superficial dermis (, ). Direct immunofluorescence of the skin lesions showed IgA (−), IgG (−), IgM (−), C3 (+, linear deposition in the basement membrane) (). The pathological diagnosis was consistent with bullous pemphigoid. Based on the clinical manifestations, serological examination, pathology, and immunofluorescence examination, a definitive diagnosis of bullous pemphigoid (moderate) was established.2B,C
Figure 2. The histopathological findings obtained from this patient. A, B: the vesicular skin lesions exhibited subepidermal blisters containing neutrophils and eosinophils, along with an infiltration of lymphocytes and eosinophils in the superficial dermis (H&E, A: ×200; B: ×400). C, D: the psoriasis lesions are characterized by hyperkeratosis and parakeratosis of the epidermis, clubbing hyperplasia of the epidermis, neutrophil infiltration, thinning of the granular layer, regular thickening of the spinous layer, and a small number of lymphocytes surrounding the blood vessels in the superficial dermis (H&E, C: ×200; D: ×400).
Treatment: The patient was administered a daily intravenous infusion of Methylprednisolone sodium succinate at a dosage of 40 mg as part of the treatment regimen. After one week of therapy, no new blisters were observed in the patient. Consequently, the medication regimen was switched to oral methylprednisolone tablets, at a daily dose of 40 mg. After undergoing therapy for four days, there was a notable improvement in the condition. This was demonstrated by the decrease in the initial erythema, the drying and formation of crusts on the blisters, and a decrease in the discharge from the eroded areas. Subsequently, the patient was discharged for outpatient follow-up while continuing prednisone orally (40 mg per day).
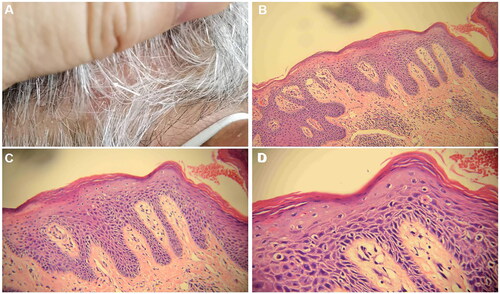
After a two-week period following discharge, the patient developed a novel skin rash accompanied by pronounced pruritus and abdominal distension, exhibiting reluctance toward glucocorticoid usage. Currently, it has been reported that biological agents like dupilumab can be employed in cases of bullous pemphigoid (BP). The EADV-recommended guidelines for bullous pemphigoid treatment also suggest dupilumab as a viable choice for patients who often exhibit a suboptimal response to treatment (Citation13). This approach facilitates faster clinical remission and aids in reducing corticosteroid dosage without any reported adverse reactions among patients utilizing dupilumab (Citation14,Citation15). Following comprehensive communication with the patient, oral methylprednisolone tablets were administered at a daily dose of 28 mg, combined with subcutaneous injections of dupilumab twice monthly, at a strength of 300 mg each time. During this treatment course, the methylprednisolone dosage was gradually reduced by 4 mg per week. When the methylprednisolone dose was reduced to 12 mg, the patient’s BP lesions were effectively controlled during the follow-up visit (). However, erythema, accompanied by overlying scales and psoriasis-like rashes, emerged on the scalp and lower limbs (). The scalp lesions were collected for pathological assessment (). A subsequent pathological examination identified hyperkeratosis accompanied by parakeratosis, epidermal thinning, and a reduction in epidermal processes. The granular layer disappeared while the dermal papillary layer moved upward. Additionally, dilated and congested blood vessels exhibited extravasation of red blood cells. Infiltration of numerous lymphocytes and plasma cells surrounding superficial dermal blood vessels was observed alongside a small number of neutrophils (). Consequently, a diagnosis of psoriasis was made.
Figure 3. A, E: The occurrence of psoriatic lesions was observed subsequent to the administration of dupilumab for a period of two weeks (PASI = 1.6). B, F: the lesions associated with psoriasis became more severe subsequent to the implementation of a dupilumab treatment regimen lasting six weeks (PASI = 2.4). C, G: following a 4-week course of treatment consisting exclusively of upadacitinib, a significant improvement in cutaneous lesions was observed, with no occurrence of new lesions (PASI = 0.8). D, H: Existing lesions continued to diminish and no new lesions were observed following a sustained 10-week course of upadacitinib treatment (PASI = 0).
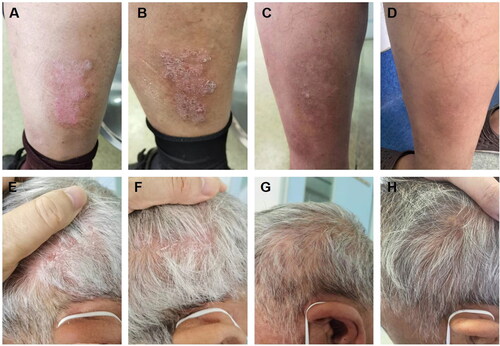
Figure 4. Histopathological findings of scalp psoriasis lesions (hematoxylin-eosin staining was utilized for histopathology). A. The samples were obtained from cutaneous lesions on the scalp that exhibited erythema and scaling. B, C, D: a small number of lymphocytes encircle the blood vessels in the superficial dermis, where hyperkeratosis and parakeratosis of the epidermis are observed, along with clubbing hyperplasia of the epidermis, neutrophil infiltration, thinning of the granular layer, and regular thickening of the spinous layer. The magnification factors for B, C, and D are 10×, 20×, and 40×, respectively.
After conducting a literature review and excluding any contraindications, the patient’s condition, risks, and costs were effectively communicated prior to obtaining informed consent for oral upadacitinib treatment at a daily dosage of 15 mg. Following two weeks of treatment, effective control of BP rash was achieved and gradual subsidence of psoriatic lesions was observed. Continued administration for four weeks resulted in significant improvement in both BP and psoriatic lesions (; ). At the conclusion of a ten-week treatment period, complete resolution without recurrence was attained for both BP and psoriatic lesions (; ). After undergoing treatment, the size of the bullous pemphigoid lesion diminishes to less than 5% of the entire body surface area. Additionally, the effectiveness of psoriasis therapies can be detected up to a PASI100. The patient is currently being regularly monitored and evaluated.
3. Discussion
Janus kinases (JAKs) are a group of cytoplasmic tyrosine kinase proteins consisting of four intracellular tyrosine kinases (JAK1, JAK2, JAK3, TYK2) that bind to the cytoplasmic domain of transmembrane cytokine receptors and regulate signal transduction. JAKs play a crucial role in the JAK/STAT signaling pathway and are involved in the signal transduction of various cytokines such as interferon (IFN), interleukin (IL)-2, IL-4, IL-7, IL-9, IL-13, IL-15, IL-21, and IL-23 (Citation16). Moreover, the JAK/STAT pathway is indispensable for T helper 1 (Th1) and Th2 cell differentiation (Citation17). Consequently, targeting JAK has gained significant attention for treating autoimmune diseases. Upadacitinib is a second-generation highly selective JAK inhibitor that specifically targets the JAK1 enzyme. By inhibiting both the JAK1 and JAK1/JAJ3 pathways simultaneously, it prevents inflammatory cytokines from utilizing the JAJ/STAT pathway while enhancing cellular immune function (Citation18).
Psoriasis and bullous pemphigoid (BP) are both prevalent skin diseases in clinical practice; however, their co-occurrence is exceedingly rare. Since the retrospective study conducted by Grattan CE et al. in 1985 (Citation19), which suggested a potential correlation between BP and psoriasis, cases of concurrent BP and psoriasis have gradually garnered attention (Citation20). Systemic glucocorticoid administration is the primary choice for managing BP, while caution must be exercised to avoid systemic glucocorticoid usage in psoriasis treatment to prevent disease exacerbation or progression into pustular or erythrodermic forms during corticosteroid tapering. Consequently, treating patients with simultaneous BP and psoriasis poses significant challenges. Therefore, identifying effective and reliable therapeutic options holds immense practical significance.
The pathogenesis of bullous pemphigoid (BP) remains unclear, and the disease is currently considered a Th1/Th2 cell-mediated disorder with dominance of Th2 cells and association with the production of BP180/BP230 antibodies (Citation21). Increasing evidence suggests that BP is closely linked to type 2 inflammation mediated by Th2 cells and type 2 cytokines, particularly IL-4 and IL-13 (Citation1). Dupilumab, a signaling biological agent inhibiting IL-4 and IL-13, has demonstrated successful efficacy in BP treatment (Citation14,Citation15). Literature also indicates the potential involvement of Th17 in BP pathogenesis through recruitment of inflammatory cells via secretion of various cytokines including IL-17, IL-21, IL-22, IFN-γ, GM-CSF etc., thereby stimulating release of proinflammatory cytokines and proteases leading to inflammatory damage (Citation1). Previous studies have demonstrated varying degrees of expression of JAK/STAT-related proteins in BP and dermatitis herpetiformis (Citation22). The JAK/STAT pathway may serve as a downstream signal for IL-4, IL-13, IL-17, and other factors, participating in the differentiation and function of Th2 and Th17 cells, thereby influencing the pathogenesis of BP. Furthermore, successful treatment of BP with JAKi provides additional support for the correlation between the JAK/STAT pathway and BP (Citation6,Citation7,Citation23).
The pathogenesis of psoriasis is intricate and not fully comprehended. Currently, it is postulated that the inflammatory response in psoriasis primarily stems from T cells, including Th1, Th17, Tc1, Tc17 cells, etc., with Th17 cells playing a pivotal central role. Cytokines TNF-α, IL-17 and IL-23 exert significant regulatory effects on the function and differentiation of Th17 cells (Citation24). The IL23/Th17 axis represents a crucial link in the pathogenesis of psoriasis. IL-23 can bind to both type I and type II cytokine receptors, activating intracellular signaling mediated by JAKs to drive Th17 cell differentiation while promoting production of IL-17 and IL-22. This leads to keratinocyte proliferation and formation of characteristic psoriatic features (Citation8). Furthermore, the JAK/STAT pathway has been implicated at various key points in the pathophysiological process of psoriasis by inducing proliferation of both Th17 cells and keratinocytes. Increasing evidence suggests that drugs targeting the JAK/STAT pathway such as JAK inhibitors (JAKi) have been utilized for treating psoriasis with promising outcomes (Citation25).
These findings suggest that the upregulation of Th17 cell function and the dysregulation of Th1 and Th2 cell function may represent common pathways in the pathogenesis of both diseases, offering potential therapeutic targets for managing bullous pemphigoid (BP) combined with psoriasis. Consequently, targeting key signaling pathways involved in the differentiation and function of Th17, Th1 cells, or other pathogenic cells like Th2 could potentially impede disease progression, thereby providing theoretical benefits to patients with BP combined with psoriasis by blocking these signaling pathways. The JAK/STAT pathway exerts regulatory control over the differentiation and function of Th17, Th1, and Th2 through various downstream cytokine signals, thus influencing the onset and progression of both diseases. By downregulating the JAK/STAT pathway, JAK inhibitors can exert a therapeutic effect on these two conditions. Upadacitinib has received FDA approval for the treatment of Rheumatoid Arthritis, Psoriatic Arthritis, Atopic Dermatitis, Ulcerative Colitis, Crohn’s Disease, Ankylosing Spondylitis and Non-Radiographic Axial Spondyloarthritis (Citation26). Additionally, case studies have reported on the potential efficacy of upadacitinib in managing bullous pemphigoid (Citation11) and nail psoriasis (Citation12).
In this case, the patient presented with bullous pemphigoid as the primary clinical manifestation upon admission, without any evident psoriatic skin lesions. Following routine anti-inflammatory treatment with methylprednisolone (40 mg qd), corticosteroid-related side effects emerged during maintenance therapy, leading to a modification in the treatment regimen to subcutaneous injection of dupilumab (300 mg) while gradually tapering off steroids. Psoriasis recurred during this process. After conducting a literature review and obtaining informed consent, oral upadacitinib was administered to the patient. During follow-up, the patient’s bullous pemphigoid lesions were effectively controlled without recurrence of psoriasis. Our case study and literature review highlight the significance of JAK inhibitors in managing both psoriasis and autoimmune bullous diseases. Compared to immunosuppressive agents and biological therapies, JAK inhibitors like upadacitinib offer ease of administration along with satisfactory efficacy and high compliance among patients. This study provides valuable clinical insights into the potential of upadacitinib as a therapeutic option for bullous pemphigoid complicated by psoriasis; however, further investigation is warranted to assess its long-term safety, efficacy, and underlying mechanisms.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fang H, Li Q, Wang G. The role of T cells in pemphigus vulgaris and bullous pemphigoid. Autoimmun Rev. 2020;19(11):1. doi: 10.1016/j.autrev.2020.102661.
- Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–6. doi: 10.1111/bjd.18245.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–744. doi: 10.1016/j.jaad.2016.12.005.
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10(December):2847. doi: 10.3389/fimmu.2019.02847.
- Xin P, Xu X, Deng C, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 2020;80(October 2019):106210. doi: 10.1016/j.intimp.2020.106210.
- Sarny S, Hucke M, El-Shabrawi Y. Treatment of mucous membrane pemphigoid with Janus kinase inhibitor baricitinib. JAMA Ophthalmol. 2018;136(12):1420–1422. doi: 10.1001/jamaophthalmol.2018.3789.
- Fan B, Wang M. Tofacitinib in recalcitrant bullous pemphigoid: a report of seven cases. Br J Dermatol. 2023;188(3):432–434. doi: 10.1093/bjd/ljac078.
- Kvist-Hansen A, Hansen PR, Skov L. Systemic treatment of psoriasis with JAK inhibitors: a review. Dermatol Ther (Heidelb). 2020;10(1):29–42. doi: 10.1007/s13555-019-00347-w.
- Xiao Y, Xiang H, Li W. Concurrent bullous pemphigoid and plaque psoriasis successfully treated with Janus kinase inhibitor baricitinib. Dermatol Ther. 2022;35(10):e15754. doi: 10.1111/dth.15754.
- Li H, Wang H, Qiao G, et al. Concurrent bullous pemphigoid and psoriasis vulgaris successfully treated with Janus kinase inhibitor tofacitinib: a case report and review of the literature. Int Immunopharmacol. 2023;122(June):110591. doi: 10.1016/j.intimp.2023.110591.
- Nash D, Kirchhof MG. Bullous pemphigoid treated with Janus kinase inhibitor upadacitinib. JAAD Case Rep. 2023;32:81–83. doi: 10.1016/j.jdcr.2022.12.006.
- Wang N, Yang Q, Liu Y, et al. Upadacitinib in nail psoriasis: a case report. J Dermatolog Treat. 2023;34(1):2246604. doi: 10.1080/09546634.2023.2246604.
- Borradori L, Van Beek N, Feliciani C, et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2022;36(10):1689–1704. doi: 10.1111/jdv.18220.
- Abdat R, Waldman RA, de Bedout V, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52. doi: 10.1016/j.jaad.2020.01.089.
- Zhang X, Man X, Tang Z, et al. Dupilumab as a novel therapy for bullous pemphigoid. Int J Dermatol. 2023;62(4):e263–e266. doi: 10.1111/ijd.16525.
- Pesu M, Laurence A, Kishore N, et al. Therapeutic targeting of Janus kinases. Immunol Rev. 2008;223(1):132–142. doi: 10.1111/j.1600-065X.2008.00644.x.
- Muddebihal A, Khurana A, Sardana K. JAK inhibitors in dermatology: the road travelled and path ahead, a narrative review. Expert Rev Clin Pharmacol. 2023;16(4):279–295. doi: 10.1080/17512433.2023.2193682.
- Duggan S, Keam SJ. Upadacitinib: first approval. Drugs. 2019;79(16):1819–1828. doi: 10.1007/s40265-019-01211-z.
- Grattan CEH. Evidence of a link between bullous pemphigoid and psoriasis. Br J Dermatol. 1985;113(3):281–283. doi: 10.1111/j.1365-2133.1985.tb02079.x.
- Kridin K, Ludwig RJ, Schonmann Y, et al. The bidirectional association between bullous pemphigoid and psoriasis: a population-based cohort study. Front. Med. 2020;7(August):1–7. doi: 10.3389/fmed.2020.00511.
- Genovese G, Di Zenzo G, Cozzani E, et al. New insights into the pathogenesis of bullous pemphigoid: 2019 update. Front Immunol. 2019;10(JUL):1506. doi: 10.3389/fimmu.2019.01506.
- Juczynska K, Wozniacka A, Waszczykowska E, et al. Expression of the JAK/STAT signaling pathway in bullous pemphigoid and dermatitis herpetiformis. Mediators Inflamm. 2017;2017:6716419–6716412. doi: 10.1155/2017/6716419.
- Kalantari Y, Sadeghi S, Asadi D, et al. A literature review on Janus kinase (JAK) inhibitors for the treatment of immunobullous disorders. Int Immunopharmacol. 2022;110(April):108923. doi: 10.1016/j.intimp.2022.108923.
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi: 10.1016/S0140-6736(20)32549-6.
- Gómez-García F, Gómez-Arias PJ, Montilla-López A, et al. A scoping review on use of drugs targeting the JAK/STAT pathway in psoriasis. Front Med. 2022;9(February):1–10. doi: 10.3389/fmed.2022.754116.
- Alten R, Mischkewitz M, Stefanski AL, et al. Janus kinase inhibitors: state of the art in clinical use and future perspectives. Z Rheumatol. 2020;79(3):241–254. doi: 10.1007/s00393-020-00768-5.

