Abstract
Purpose: Topical treatments for mild-to-moderate (MM) atopic dermatitis (AD) include emollients, corticosteroids, calcineurin inhibitors, a Janus kinase inhibitor, and a phosphodiesterase 4 inhibitor, which differ in multiple ways. This study aimed to quantify the conditional relative importance (CRI) of attributes of topical treatments for MM AD among adult and adolescent patients and caregivers of children with MM AD.
Materials and methods: A discrete-choice experiment (DCE) survey was administered to US adults and adolescents with MM AD and caregivers of children with MM AD. Each choice task comprised 2 hypothetical topical treatments characterized by efficacy, adverse events, vehicle, and application frequency. Data were analyzed using a random-parameters logit model to calculate the CRI of each attribute.
Results and conclusions: 300 adults, 331 adolescents, and 330 caregivers completed the DCE. Avoiding changes in skin color (CRI 29.0) and time until itch improves (26.6) were most important to adults, followed by time until clear/almost clear skin (17.8). Application frequency (3.0) did not have a statistically significant impact on adults’ choices. Adolescents were less concerned about changes in skin color than adults or caregivers; caregivers were less concerned about time until clear/almost clear skin than patients. Physicians should consider age-relevant aspects of preferences in treatment discussions with patients and caregivers.
Introduction
Atopic dermatitis (AD), characterized by intense itching, dry skin, redness, and exudation (Citation1–6), is a very common skin condition. AD is more prevalent in children and adolescents (15%) than in adults (3%–5%) (Citation7) and is associated with substantial economic and quality-of-life burden (Citation8–10). Topical agents, a mainstay of treatment for mild-to-moderate AD, include emollients (e.g., creams, lotions, ointments), corticosteroids (e.g., hydrocortisone), calcineurin inhibitors (e.g., tacrolimus, pimecrolimus), a phosphodiesterase 4 (PDE 4) inhibitor (crisaborole), and a Janus kinase inhibitor (ruxolitinib) (Citation2,Citation10–14). These topical treatments differ in clinical (i.e., efficacy and safety) and nonclinical attributes as well as formulation.
Because nonclinical outcomes often cannot be captured in traditional trials or cost-effectiveness analysis, understanding individuals’ preferences can help inform shared decision-making for physicians and provide crucial insight for regulatory and payer discussions (Citation15,Citation16). Furthermore, given that no treatment option for mild-to-moderate AD is clearly superior to all the others, treatment choice is a preference-sensitive decision (Citation17). While previous preference research suggests that efficacy, safety, and mode of administration are all important treatment considerations for adult patients with AD (Citation18–20), preferences for topical treatments for mild-to-moderate AD among adults and adolescents and caregivers of children with AD have not been well characterized.
This study aimed to quantify the relative importance of treatment attributes among adults, adolescents, and caregivers using a discrete-choice experiment (DCE) survey instrument.
Materials and methods
An online DCE survey was administered to US adults and adolescents with mild-to-moderate AD and caregivers of children with mild-to-moderate AD to elicit preferences for treatment attributes. The RTI International institutional review board (IRB) reviewed and approved the study (IRB ID number STUDY00021508) on 4 April 2022. All adult survey respondents provided electronic informed consent to participate in the survey, and adolescent respondents provided assent to participate, with the parent’s permission.
Study population
Adults (aged ≥ 18 years) and adolescents (aged 12–17 years) with a self-reported physician diagnosis of mild-to-moderate AD and caregivers of children (aged 3 months to 11 years) with a self-reported physician diagnosis of mild-to-moderate AD meeting the study selection criteria were recruited by Global Perspectives, a healthcare research firm. Caregiver respondents who had an adolescent (aged 12–17 years) with AD were also asked if the adolescent would be able to participate in the study. The study team aimed to recruit each cohort so that approximately half of participants had mild and half had moderate AD. All respondents were able to read and understand English.
Survey development
Survey development was informed by a previously conducted study that involved in-depth, one-on-one qualitative research interviews. The final set of attributes included in the DCE () thus reflected the priorities of people with AD as well as of caregivers of children with AD (Citation21). The treatment attributes also differentiated among currently available topical treatments and those in development.
Table 1. Attributes and levels for the discrete-choice experiment.
The survey instrument also included questions about respondents’ demographic characteristics and experiences using topical treatments for mild-to-moderate AD, questions to assess respondent comprehension of the treatment attributes, DCE questions, and three patient-reported outcome (PRO) instruments (the Patient-Oriented Eczema Measure [POEM] (Citation22,Citation23), the 24-h Peak Pruritus Numerical Rating Scale [NRS] (Citation24,Citation25), and the Topical Corticosteroid Phobia [TOPICOP] scale (Citation26)). The POEM, a patient-reported symptom scale measuring the severity of AD over the previous week, was used to screen respondents and categorize AD as mild, moderate, or severe. Respondents with severe AD, as determined by the POEM, were asked follow-up questions about their treatment history to determine whether they would be categorized as having moderate AD for this study; if their treatment history included severe AD treatments, they were ineligible for the study. The Peak Pruritus NRS assessed itch severity at its worst moment in the previous 24 h on a scale from 0 (‘no itch’) to 10 (‘worst itch imaginable’).
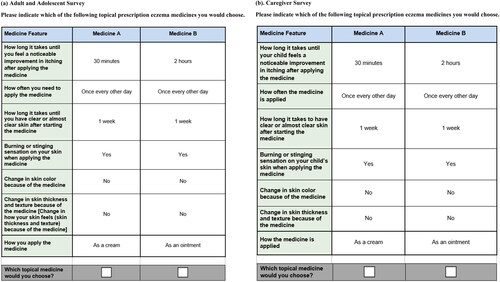
In the DCE portion of the survey, respondents chose between alternative hypothetical treatment profiles in a series of choice questions about drugs labeled only as ‘Medicine A’ and ‘Medicine B’ (i.e., no drug names were provided) (). Each hypothetical treatment profile was defined by attributes with varying levels (). The profiles and profile pairs in each choice question were determined by an experimental design, which included 72 hypothetical treatment pairs split into 6 blocks of 12 hypothetical choice questions. Each respondent was randomly assigned to 1 block of questions; questions were randomly ordered to avoid ordering effects and were not repeated across blocks.
Figure 1. Example discrete-choice experiment questions. (a) Adult and adolescent survey. Please indicate which of the following topical prescription eczema medicines you would choose. (b) Caregiver survey. Please indicate which of the following topical prescription eczema medicines you would choose. Note: Each respondent was presented with 12 choice tasks based on an experimental design of 72 choice tasks, divided into 6 blocks.
The survey instrument was pretested during 45 in-person cognitive interviews (conducted with a convenience sample of 15 participants for each cohort included in the study population) to confirm that the questions were comprehensible and that the attributes and levels were comprehensive, relevant, and appropriately described.
Statistical analyses
The DCE data for each cohort of respondents (i.e., adults, adolescents, and caregivers) were analyzed using separate random-parameters logit (RPL) models following good research practices (Citation27). This model relates respondents’ choices to the attribute levels included in the DCE experimental design and estimates a set of relative preference weights for the attribute levels included in a DCE survey. The RPL model accounts for the panel nature of the data and for unobserved differences in preferences across respondents by assuming a distribution for each estimated preference weight. The model output was used to calculate the conditional relative importance (CRI) of each attribute included in the DCE. The CRI is a measure of the importance of each attribute, conditional on the range of levels of that attribute, relative to the importance of all other attributes in the study. The CRI for each attribute is calculated as the difference between the preference weight for the most-preferred and least-preferred level. The CRIs were rescaled such that they sum to 100 within each cohort, so that we can interpret each one as the proportion of utility that can be gained by improving each attribute from the least- to the most-preferred level relative to the maximum utility that can be gained in each cohort by improving every attribute in the same way. The standard errors and 95% CIs for these differences are calculated using the delta method (Citation28,Citation29). Finally, descriptive statistics were computed for all questions, and the relationships between respondents’ scores on the PRO measures were explored using linear regression analysis (see ). All analyses were conducted using STATA 17 (Stata Corp, College Station, TX).
Results
Respondent characteristics
A total of 300 adults and 331 adolescents with mild-to-moderate AD and 330 caregivers of children with mild-to-moderate AD completed the survey and were included in the final analysis sample (). Of the adult cohort, 69% of respondents were female, and mean age was 53 years. Most adult respondents self-identified as White (90%), while approximately 7% self-identified as Black or African American (not Hispanic or Latino) and 4% as Hispanic or Latino. Of the adolescent cohort, 48% of respondents were female, with a mean age of 15 years. Most adolescent respondents self-identified as White (78%), approximately 13% as Black or African American (not Hispanic or Latino), and 10% as Hispanic or Latino. Of the caregiver cohort, 74% of respondents were female, with a mean age of 36 years. Most caregiver respondents self-identified as White (72%), approximately 18% as Black or African American (not Hispanic or Latino), and 11% as Hispanic or Latino.
Table 2. Respondent demographic and treatment characteristics.
Mean POEM scores across cohorts ranged from 9.3 to 10.5, of a maximum score of 28 (with higher scores indicating more severe AD; ). Mean Peak Pruritus NRS ranged from 5.3 to 5.6, of a maximum score of 10 (with higher scores indicating more severe itch). Mean TOPICOP scores ranged from 50.8 to 54.2, of a maximum score of 100 (with higher scores indicating greater concern about taking topical steroids). Compared with data from US clinical practice, mean results for each of the three cohorts suggest that, on average, respondents had moderately severe eczema (by the POEM) (Citation22) and moderate pruritus (by Peak Pruritus NRS) (Citation30). Topical corticosteroid phobia (by TOPICOP) was rather high compared with data from US and ex-US clinical practice (Citation31,Citation32).
Table 3. Respondent patient-reported outcome summary scores.
Preference weights
For attributes with a natural ordering of levels (time until itching relief, time to clear or almost clear skin, and adverse events [AEs]), the preference weights were ordered as expected in all cohorts, with better outcomes being preferred to worse outcomes (). On average, adult respondents preferred creams to ointments, but were indifferent between different frequencies of administration. Adult respondents were also indifferent in the choice between one week versus three weeks until clear or almost clear skin (p = .102) and one week versus four weeks for the same (p = .155). However, four weeks until clear or almost clear skin was strongly preferred to eight weeks. Differences in preference weight estimates can be compared within and across attributes to describe relative preferences for changes in different attribute levels. For example, a reduction in time until itch relief from 12 h to 6 h was approximately 1.2 times more important than a change in formulation from ointment to cream ([−0.599 − (−0.266)] ÷ [−0.137 − 0.137]) = 1.215).
Figure 2. Random-parameters logit model estimates. (a) Adult preference weights (N = 300). (b) Adolescent preference weights (N = 331). (c) Caregiver preference weights (N = 330). DCE: discrete-choice experiment. a ‘Formulation’ was described in patient-friendly language as ‘mode of administration’ in the survey. Notes: Attributes are presented in the order in which they appeared in the DCE questions. The vertical bars around each mean preference weight represent the 95% confidence interval around the point estimate. Because all attribute levels are effects coded, the sum of preference weights across levels of an attribute equals 0. Within each attribute, a higher preference weight indicates that a level is more preferred. For example, on average, adult respondents preferred itch relief in 30 min (weight = 0.663) more than itch relief in 6 h (weight = −0.266). The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the preference weights for any 2 levels of that attribute. Larger differences between preference weights indicate that respondents viewed the change as having a relatively greater effect on overall utility.
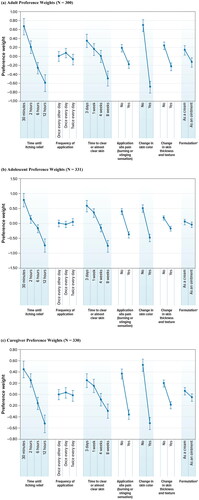
On average, adolescent respondents were indifferent between levels of frequency of administration and mode of administration. Adolescent respondents were also indifferent in the choice between one week versus three days until clear or almost clear skin. A comparison within and across attributes to describe relative preferences for changes in levels shows that a reduction in time until itching relief from 12 h to 30 min was approximately 1.6 times more important than avoiding a change in skin color caused by topical medicine ([−0.746 − 0.781] ÷ [−0.492 − 0.492]) = 1.552).
On average, caregivers were indifferent between frequencies of administration and modes of administration. Caregivers were also indifferent in the choice between 2 h versus 30 min until itching relief (p = .063) and between three days versus one week until clear or almost clear skin (p = .332). A comparison within and across attributes to describe relative preferences for changes in levels demonstrates that avoiding a change in skin color was approximately 1.9 times more important to caregivers than a reduction in time until clear or almost clear skin from eight weeks to three days ([−0.519 − 0.519] ÷ [−0.299 − 0.246]) = 1.905).
Conditional relative importance
presents CRI estimates by cohort. For adults, avoiding a change in skin color (CRI, 29.0) and time until itch improves (CRI, 26.6) were most important when choosing between hypothetical treatments, followed by time to clear or almost clear skin (CRI, 17.8). Avoiding changes in skin thickness and texture (CRI, 9.8), avoiding application site pain (CRI, 7.9), and formulation (CRI, 5.8) were the next-most-important attributes. Frequency of administration (CRI, 3.0) was the least important attribute and did not have a statistically significant impact on adults’ treatment choice.
Figure 3. Random-parameters logit model estimates: conditional relative attribute importance. ASP: application site pain; CRI: conditional relative importance; DCE: discrete-choice experiment. a ‘Formulation’ was described in patient-friendly language as ‘mode of administration’ in the survey. Notes: Attributes are presented in the order in which they appeared in the DCE questions. The CRI is the difference between the preference weights on the most influential attribute level and the least influential attribute level. These differences are summed across attributes, and the sum is scaled to 100. The conditional importance of each attribute is a percentage of this total. The standard errors and 95% confidence intervals for these differences were calculated using the delta method. The 95% confidence interval around the point estimate is represented by the black vertical bars on top of the colored bars.
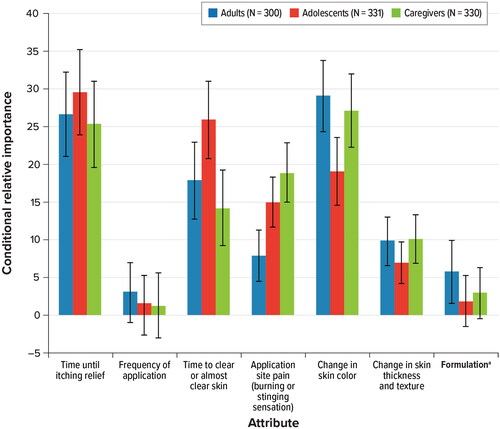
Adolescent patients were much less concerned about avoiding a change in skin color (CRI, 19.1) than adult patients (CRI, 29.0) or caregivers (CRI, 27.1). Compared with adult (CRI, 17.8) and adolescent patients (CRI, 25.9), caregivers (CRI, 14.2) were less concerned about time to clear or almost clear skin, but caregivers were more concerned with application site pain (CRI, 18.9) than adults (CRI, 7.9) or adolescents (CRI, 15.0). Frequency of application was not identified as the most important treatment attribute for any of the cohorts.
Relationship between PRO scores
presents results from the linear regression for the TOPICOP, including only the POEM (regression 1), only the Peak Pruritus NRS (regression 2), and both the POEM and the Peak Pruritus NRS (regression 3), and the linear regression for the POEM on the Peak Pruritus NRS (regression 4). The regression coefficients indicate the effect of a one-unit increase in the explanatory variable on the dependent variable. For example, regression 1 suggests that, for adults, a 1-unit increase in POEM score (increase in AD severity) is associated with an approximately 0.8-unit increase in TOPICOP score.
Table 4. Linear regression of outcome PRO variables against selected explanatory variables by cohort.
The regression analyses revealed that, for adults and caregivers, the POEM and Peak Pruritus NRS scores were statistically significantly positively correlated, suggesting that increases in itch severity and AD severity were associated with increases in topical steroid phobia as reflected by TOPICOP score among respondents. For all 3 cohorts, the POEM and Peak Pruritus NRS were statistically significantly correlated.
Discussion
This quantitative study, informed by qualitative research, elicited preferences for attributes of current and pipeline mild-to-moderate AD treatments and quantified the relative importance of these treatment attributes for US adults and adolescents with mild-to-moderate AD and caregivers of children with mild-to-moderate AD (Citation21). For all three cohorts, the preference weights for attributes with a natural ordering (time until itching relief, time to clear or almost clear skin, and AEs) were ordered as expected, with better outcomes being preferred to worse outcomes. Adults preferred a treatment applied as a cream rather than an ointment, but adolescents and caregivers did not have a statistically significant preference between modes of administration. The estimates of CRI of attributes (i.e., the difference between the most-preferred and least-preferred levels of an attribute) were similar across cohorts, but caregivers were more concerned with application site pain than were patients (adults and adolescents). Compared with adults and caregivers, adolescents with mild-to-moderate AD were less concerned about changes in skin color. The CRI estimates for frequency of administration were not significant for any of the cohorts, indicating that frequency of administration had no statistically significant impact on treatment preferences for patients or caregivers.
Adults, adolescents, and caregivers were all very concerned about time until itching relief. This finding is consistent with prior evidence that pruritus is the most burdensome AD symptom for patients, resulting in impaired quality of life, sleep disturbances, and emotional impacts including anxiety and depression (Citation33–35). In a survey of 1678 patients with AD in Germany, the most commonly reported treatment needs were freedom from pruritus (96%) and getting better skin quickly (88%) (Citation36). Together these results emphasize that rapid, effective relief of pruritus is a consistent priority for patients with AD and their caregivers.
Concern about application site pain varied among the cohorts. Caregivers most strongly preferred avoiding application site pain, followed by adolescents, and then followed by adults. This finding is intuitive, in that caregivers are reluctant to provide their child with a painful treatment (Citation37). Moreover, while distinct from application site pain as a side effect of treatment, AD-related skin pain is known to correlate with AD severity and pruritus, as well as with AD activity in the plantar, chest, and palmar areas, revealing the complex and multifaceted symptom burden of AD (Citation38).
Adults and caregivers were more concerned about changes in skin color than adolescents. Skin discoloration may be caused not only by steroidal treatments for AD but also by AD itself. Respondents were shown images of patients experiencing skin discoloration consistent with vitiligo, and the severe manifestation of skin discoloration shown in this example may have influenced their concern about this attribute. Nonetheless, the ordering of the other attributes is not affected even if the skin discoloration attribute is not considered. In addition, the finding that respondents did not prioritize frequency of application was a somewhat surprising result. Qualitative evidence suggests that administration frequency is an important feature of topical therapy but that the administration schedule as indicated is not a central consideration in patients’ use of these agents (Citation21). While some patients tend to use topical therapies implicitly on an as-needed basis, rather than at regular intervals as indicated to prevent disease, prior qualitative evidence indicates that patients generally prefer less-frequent application and a longer duration of effectiveness.
The preference research conducted to date in AD has focused on adult patient populations (Citation18–20) and has primarily evaluated the attributes of therapies used in moderate to severe discase (Citation18,Citation19). A DCE study of systemic treatments conducted among US individuals with moderate-to-severe AD found that avoiding serious risks (including serious infection, venous thromboembolism, and, especially, malignancy), better skin clearance, shorter time to itch relief, and daily oral dosing (vs. biweekly injections) were treatment priorities for patients (Citation18). In a DCE comparing the preferences of patients with moderate-to-severe AD and dermatologists in Japan, dermatologists were more concerned than patients with efficacy in treating rashes and treatment costs, whereas patients preferred add-on over replacement therapy and were averse to self-administered injections (Citation19). Most recently, a DCE evaluated US adult patients’ preferences for attributes of topical, oral, and injectable therapies for mild-to-moderate AD (Citation20). Adult patients most prioritized probability of clear skin after 16 weeks, followed by reducing risk of serious infection, topical over injectable administration, reducing time until itch improves, and reducing risk of application site pain. Our study is the first DCE to have evaluated and compared the preferences of adult patients, adolescent patients, and caregivers for attributes associated specifically with topical therapies for mild-to-moderate AD.
Some limitations of this study are acknowledged. Respondents were recruited through a research panel and may not be representative of the broader US AD population (particularly with respect to age) or of caregivers of children with AD. Diversity with respect to race/ethnicity and education was limited: most of the sample identified as White (72.1%–89.7% across cohorts), and 83.0% of adults and 77.6% of caregivers had above a high-school education. The results therefore are subject to potential volunteer bias, information bias, and recruitment bias. All data, including AD diagnosis, severity, and treatment history, were self-reported and not validated by clinician review or review of medical records. Related to treatment history, respondents had not been asked to describe the extent of long-term remission time that may have been achieved with topical therapy, which may have impacted their responses for the DCE. Respondents were asked to make hypothetical choices, which might not predict actual decisions made in a clinical setting. Not all attributes of topical therapies for mild to moderate AD were included in the DCE survey, and not all adverse-event attributes that were included will occur for patients using topical therapies. The average preferences reflected in our results are conditional on the range of attributes and levels evaluated; preferences may have differed had other attributes been included. Nonetheless, the DCE has a number of strengths. Respondents’ mean POEM and Peak Pruritus NRS scores were comparable with those of the broader population with moderate AD severity, and mean TOPICOP scores were consistent with the US AD population, supporting the representativeness of the sample in terms of AD severity (Citation22,Citation30,Citation32). In particular, the survey was qualitatively pretested and subsequently refined, and both the survey and its experimental design were carefully developed according to best practices (Citation39,Citation40). The treatment-choice data were analyzed using advanced RPL methods following good research practices (Citation27,Citation41) that avoid both estimation bias from unobserved variation in preferences across the sample and within-sample correlation in the choice sequence for each respondent.
In conclusion, this study demonstrates, in a quantitative manner through direct subject self-reports across a sample of 961 subjects, that adults, adolescents, and caregivers affected by AD consistently place the highest value on rapid symptom control and minimizing side effects, as may be expected; however, the strength of preferences for specific attributes, interestingly, varies widely across the three cohorts. Notably, caregivers prioritize minimizing application site pain, whereas adolescents express less concern about changes in skin color compared with adults and caregivers. It must be noted that the average preferences presented for the cohorts do not reflect individual patient and caregiver preferences, which may vary and which must be considered when developing an individualized treatment plan in partnership with patients and caregivers.
Our results highlight the importance of a nuanced approach to treatment selection for AD. When discussing AD treatment options with patients and caregivers, it is important for clinicians to consider age-relevant preferences, as well as individual preferences of both patients and caregivers. Clinicians should have tools available to assess patient and caregiver preferences and should engage in detailed discussions of treatment options to ensure that patients are receiving treatments that align with their preferences and values. For drug developers, prioritizing therapies with rapid itch relief and minimal side effects, offered in patient-preferred application forms, is crucial. Further research exploring heterogeneity in preferences within patient and caregiver cohorts, as well as for different treatment types and AD severities, may contribute to more personalized and effective AD management.
Consent form
The participants in this study provided written informed consent.
Acknowledgments
Kimberly Moon of RTI Health Solutions provided overall project management for this study. Kate Lothman of RTI Health Solutions provided medical writing services during manuscript development.
Disclosure statement
This study was conducted under a research contract between Pfizer Inc. and RTI Health Solutions and was funded by Pfizer Inc. Robert Gerber, Amy Cha, Brett Hauber, Joseph C. Cappelleri, and Jason Xenakis are employees of Pfizer Inc. and may hold stock/stock options. Maureen P. Neary was an employee of Pfizer Inc. and held stock at the time of this work; she is now an employee of The Janssen Pharmaceutical Companies of Johnson & Johnson. Marco Boeri and Colton Leach were employees of RTI Health Solutions at the time of this work. Steven R. Feldman has received research, speaking, and/or consulting support from Eli Lilly and Company, GlaxoSmithKline/Stiefel, AbbVie, Janssen, Alovtech, vTv Therapeutics, Bristol-Myers Squibb, Samsung, Pfizer, Boehringer Ingelheim, Amgen, Dermavant, Arcutis, Novartis, Novan, UCB, Helsinn, Sun Pharma, Almirall, Galderma, Leo Pharma, Mylan, Celgene, Ortho Dermatology, Menlo, Merck & Co, Qurient, Forte, Arena, Biocon, Accordant, Argenx, Sanofi, Regeneron, the National Biological Corporation, Caremark, Teladoc, Eurofins, Informa, UpToDate, and the National Psoriasis Foundation. He is founder and part owner of Causa Research and holds stock in Sensal Health.
Additional information
Funding
References
- Bechman K, Yates M, Galloway JB. The new entries in the therapeutic armamentarium: the small molecule JAK inhibitors. Pharmacol Res. 2019;153:1. doi: 10.1016/j.phrs.2020.104634.
- Garside R, Stein K, Castelnuovo E, et al. The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation. Health Technol Assess. 2005;9(29):1–8. doi: 10.3310/hta9290.
- Newton L, DeLozier AM, Griffiths PC, et al. Exploring content and psychometric validity of newly developed assessment tools for itch and skin pain in atopic dermatitis. J Patient Rep Outcomes. 2019;3(1):42. doi: 10.1186/s41687-019-0128-z.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract. 2019;7(8):2699–2706.e2697. doi: 10.1016/j.jaip.2019.05.055.
- Suárez-Fariñas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127(4):954–964. doi: 10.1016/j.jaci.2010.12.1124.
- Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548 e543–552 e543. doi: 10.1016/j.anai.2017.09.076.
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–1293. doi: 10.1111/all.13401.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi: 10.1111/j.1525-1470.2005.22303.x.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the national health and wellness survey. J Am Acad Dermatol. 2017;77(2):274–279 e273. doi: 10.1016/j.jaad.2017.04.019.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023.
- Baron S, Cohen S, Archer C. Guidance on the diagnosis and clinical management of atopic eczema. Clin Exp Dermatol. 2012;37(Suppl 1):7–12. doi: 10.1111/j.1365-2230.2012.04336.x.
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10 e12–22 e12. doi: 10.1016/j.anai.2017.10.039.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–349. doi: 10.1016/j.jaad.2014.03.030.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218–1233. doi: 10.1016/j.jaad.2014.08.038.
- Johnson FR, Zhou M. Patient preferences in regulatory benefit-risk assessments: a US perspective. Value Health. 2016;19(6):741–745. doi: 10.1016/j.jval.2016.04.008.
- Mühlbacher AC, Juhnke C, Beyer AR, et al. Patient-focused benefit-risk analysis to inform regulatory decisions: the European union perspective. Value Health. 2016;19(6):734–740. doi: 10.1016/j.jval.2016.04.006.
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4(1):75. doi: 10.1186/1748-5908-4-75.
- Boeri M, Sutphin J, Hauber B, et al. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatolog Treat. 2020;33(3):1449–1458.
- Okubo Y, Ho KA, Fifer S, et al. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatolog Treat. 2020;31(8):821–830. doi: 10.1080/09546634.2019.1623860.
- Myers K, Silverberg JI, Parasuraman S, et al. Treatment preferences among patients with mild-to-moderate atopic dermatitis. J Dermatolog Treat. 2023;34(1):2215356.
- Ervin C, Crawford R, Evans E, et al. Patient and caregiver preferences on treatment attributes for atopic dermatitis. J Dermatol Treat. 2022;33(4):2225–2233.
- Charman CR, Venn AJ, Ravenscroft JC, et al. Translating patient-oriented eczema measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol. 2013;169(6):1326–1332. doi: 10.1111/bjd.12590.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519. doi: 10.1001/archderm.140.12.1513.
- Simpson E, Beck LA, Gadkari A, et al. Defining a responder on the peak pruritus numerical rating scale (NRS) in patients with moderate-to-severe atopic dermatitis: detailed analysis from randomized trials of dupilumab. J Am Acad Dermatol. 2017;76(6):AB93.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak pruritus numerical rating scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769. doi: 10.1111/bjd.17744.
- Moret L, Anthoine E, Aubert-Wastiaux H, et al. TOPICOP©: a new scale evaluating topical corticosteroid phobia among atopic dermatitis outpatients and their parents. PLoS One. 2013;8(10):e76493. doi: 10.1371/journal.pone.0076493.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004.
- Greene WH. Econometric analysis. 7th ed. Harlow: Pearson; 2012.
- Hole AR. A comparison of approaches to estimating confidence intervals for willingness to pay measures. Health Econ. 2007;16(8):827–840. doi: 10.1002/hec.1197.
- Reich A, Chatzigeorkidis E, Zeidler C, et al. Tailoring the cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97(6):759–760. doi: 10.2340/00015555-2642.
- Gerner T, Haugaard JH, Vestergaard C, et al. Healthcare utilization in Danish children with atopic dermatitis and parental topical corticosteroid phobia. Pediatr Allergy Immunol. 2020;32(2):331–341. doi: 10.1111/pai.13394.
- Stalder J-F, Aubert H, Anthoine E, et al. Topical corticosteroid phobia in atopic dermatitis: international feasibility study of the TOPICOP score. Allergy. 2017;72(11):1713–1719. doi: 10.1111/all.13189.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006.
- Ständer S, Yosipovitch G, Bushmakin AG, et al. Examining the association between pruritus and quality of life in patients with atopic dermatitis treated with crisaborole. J Eur Acad Dermatol Venereol. 2019;33(9):1742–1746. doi: 10.1111/jdv.15712.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498. doi: 10.1016/j.jaad.2015.10.043.
- Augustin M, Langenbruch A, Blome C, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol. 2020;34(1):142–152. doi: 10.1111/jdv.15919.
- Curry ZA, Johnson MC, Unrue EL, et al. Caregiver willingness to treat atopic dermatitis is not much improved by anchoring. J Dermatolog Treat. 2019;30(5):471–474. doi: 10.1080/09546634.2018.1530442.
- Thyssen JP, Halling-Sonderby AS, Wu JJ, et al. Pain severity and use of analgesic medication in adults with atopic dermatitis: a cross-sectional study. Br J Dermatol. 2020;182(6):1430–1436. doi: 10.1111/bjd.18557.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13. doi: 10.1016/j.jval.2012.08.2223.
- Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–2993. doi: 10.1007/s00464-014-4044-2.

