Abstract
Background: Little is known about the extent of impairments in work and activities of daily life (ADL) in patients with psoriasis, and the influence of contextual factors such as disease-related characteristics and treatment. Therefore, this study aimed to assess these impairments in patients with psoriasis who started using biologicals/small molecule inhibitors.
Methods: Using data from the prospective BioCAPTURE registry, we collected patient, disease, and treatment parameters, as well as work/ADL impairments at baseline, 6 and 12 months. Changes in impairment parameters and correlations between impairment and patient/disease characteristics were assessed using generalized estimating equations.
Results: We included 194 patients in our analysis. After biological initiation, disease activity decreased significantly (PASI 11.2 at baseline versus 3.9 at 12 months, p < 0.001). Work-for-pay in this cohort was lower than in the Dutch general population (53% versus 67%, p = 0.01). In patients who had work-for-pay, presenteeism improved over time (5% at baseline versus 0% at 12 months, p = 0.04). Up to half of the patients reported impairments in ADL, which did not change over time. Associations between impairments and contextual factors varied, but all impairments were associated with worse mental/physical general functioning.
Conclusion: Patients with psoriasis using biologicals are less likely to have work-for-pay. Treatment improves the work productivity of employed patients, but we were unable to detect changes in ADL performance.
Keywords:
Introduction
Psoriasis is an immune-mediated inflammatory disease of skin and nails, which can impact a patient’s life in several ways. Sensations of pain, burning, or itching can affect the physical well-being of a patient, while the stigma of (visible) skin lesions can have an impact on psychological well-being (Citation1). Moreover, treatment of psoriasis can be time-consuming (e.g. application of topicals multiple times a day, or multiple hospital visits for UV therapy) or have side effects (e.g. nausea or injection site reactions) (Citation2). All these burdens can culminate in impairments in a patient’s personal and professional daily life.
Patients with psoriasis mention that pain and fatigue disrupt their normal family roles (Citation3). Moreover, patients experience a negative influence of the disease on work performance (Citation4, Citation5). Sick leave has shown to be more common in psoriasis patients when compared to the US general population: during one year, 56% of psoriasis patients took sick leave, versus 42% of the general population (Citation6). Moreover, impairments in work and daily life activities increase with increased severity of psoriasis (Citation4, Citation7, Citation8), and diminish after successful treatment (Citation9–11).
While we know that the impact of psoriasis on work and activities of daily life (ADL) is an important theme for patients, we know little about the different areas of ADL affected by the disease (Citation12, Citation13). Also, the influence of contextual factors such as sex, relationship status, educational level, and comorbidity on these impairments of ADL is unknown. Moreover, most data on treatment effects on work and ADL impairment are based upon (secondary outcomes of) randomized clinical trials, where real-world data is lacking (Citation9, Citation14–21).
Therefore, we assessed the extent of impairments in work and ADL in a daily practice cohort of patients with plaque psoriasis treated with biologicals/small molecule inhibitors (smi). In addition, we examined the effect of 6–12 months of treatment on these impairments and explored associations between impairment and contextual factors and treatment success.
Patients and methods
Study design and population
For this study, we used data from the Continuous Assessment of Psoriasis Treatment Use Registry with Biologics (BioCAPTURE registry – www.biocapture.nl). In short, this prospective, multicenter registry records data of adult patients with plaque psoriasis using biologicals/smi from 4 academic and 17 nonacademic dermatology centers in the Netherlands. Under Dutch law, this non-interventional study is exempt from ethics review by the medical ethical committee. Informed consent was obtained from all patients before inclusion in the study, and it was performed in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki.
Data collection
We collected data from patients from inclusion in the BioCAPTURE registry from 2010–2021, with a per-patient follow-up time of one year. Patients were included for the present analysis from the start of their first biological therapy registered in BioCAPTURE on, and data were collected every three months up to one year after initiation (regardless of treatment switch within this first year). For this analysis, we used all data of patients who completed questionnaires about work participation and/or ADL impairment at baseline assessment and at least one follow-up timepoint. Patients who discontinued their biological or switched to another biological, but continued to provide data, were also included. Patients who did not provide follow-up data were excluded from the analysis.
Data collected included information about contextual factors and disease-related characteristics. Contextual factors included were age, sex, relationship status, education, and comorbidity (using the Charlson Comorbidity Index (CCI)) (Citation22). Comorbidity was further categorized into low (CCI 0 points), intermediate (CCI 1-2 points), and high (CCI 3 or more). Disease-related characteristics included were disease duration, presence of concomitant psoriatic arthritis -PsA-, current biological use, and disease activity assessed with the Psoriasis Area and Severity Index (PASI) (Citation23). Current biological use was categorized per mode of action: TNFα-inhibitors (i.e. etanercept, adalimumab, infliximab, certolizumab), IL-17 inhibitors (i.e. secukinumab, ixekizumab, brodalumab), IL23-inhibitors (i.e. guselkumab, risankizumab), IL12/23 p40 inhibitors (i.e. ustekinumab), and PDE4-inhibitors (apremilast).
Other patient-reported outcomes included skin-related quality of life assessed with the Dermatological Life Quality Index (DLQI) (Citation24), and physical and mental wellbeing assessed with the component scores of the Short Form 36 (PCS/MCS) (Citation25).
Primary outcomes were impairments in work participation and ADL. Data about work participation were collected using the PROductivity and DISease Questionnaire (PRODISQ) (Citation26). Work participation parameters were: having work-for-pay, absenteeism (percentage of time being away from work), and presenteeism (percentage of estimated “productivity loss” while at work). Absenteeism and presenteeism can be combined into overall work impairment as follows: Absenteeism + ((1-Absenteeism) * Presenteeism). All work parameters are reported in percentage of maximum work output as reported by patients, usually in median percentage reported and interquartile ranges (IQR).
Data about impairments in ADL were collected from the TIC-P questionnaire (Citation27). Patients were asked if they experienced any impairments in four ADL domains: household chores (i.e. cooking, cleaning), grocery shopping (outside of the home), home maintenance and childcare. Answers were dichotomized into ADL impairment present or not for each domain.
Statistical analysis
Continuous data were described with mean (standard deviation, SD) or median (interquartile ranges, IQR). Categorical data were described as absolute frequencies (percentages).
We used generalized estimating equations (GEE) to explore differences in disease-related and patient-reported outcomes (i.e. PASI, DLQI, PCS, MCS, work and ADL impairment) at different timepoints, and to explore associations of work/ADL impairments with disease-related characteristics and contextual factors. GEE allows the estimation of the average effect of an independent variable on a specific outcome at the population level (Citation28). For example, we can estimate the average effect of a change in PASI on the likelihood of having work-for-pay. Since GEE makes use of all available data, missing data was not imputed.
First, differences in disease-related and patient-reported outcomes between different timepoints were tested. For continuous outcomes (e.g. PASI, DLQI, presenteeism) a linear GEE model was used, while for binary outcomes (e.g. work-for-pay, ADL impairment) a logistic GEE model was used. Timepoints (baseline, 6 months – M6, 12 months – M12) were entered as independent variables. Baseline values were regarded as the default state, and statistical significance of values at M6 and M12 were tested in comparison to baseline.
Second, we assessed the extent of work impairment in the study patients. Also, we compared the work-for-pay status (proportion with paid work) of the BioCAPTURE cohort with the Dutch general population by using an age- and sex-matched model based on data from the Central Bureau of Statistics (CBS) of the Netherlands (Citation29). The CBS provides yearly data on employment rates, stratified for sex and age groups per ten years of age. Data were available from 2013 onwards. BioCAPTURE patients included before 2013 were matched to the general population of 2013. Differences between the proportions of patients with work for pay in the BioCAPTURE cohort vs. the general population were tested by a Chi-square test.
Third, we used four separate logistic GEE models to test associations of work/ADL impairments with disease-related characteristics and contextual factors. Work-for-pay (yes/no), impairment in household chores (yes/no), impairment in grocery shopping (yes/no), and impairment in home maintenance (yes/no) were the dependent variable in each of the models. To explore the influence of disease-related characteristics and contextual factors on presenteeism, we used a linear GEE model. Independent variables entered in the models were: age, sex, relationship status, education (primary/secondary versus tertiary), presence of PsA, disease duration of psoriasis, PASI over-time, DLQI over-time, MCS over-time, PCS over-time, and whether the biological/smi used at baseline was still used after 6/12 months.
Last, to assess the association of work/ADL impairments with treatment success, we compared the parameters of work/ADL impairment (work-for-pay, presenteeism, and impairments in household chores, grocery shopping, and home maintenance) at different timepoints between patients who did and did not have treatment success. As a proxy for treatment success, we used PASI ≤ 1.0 at 6/12 months, PASI ≤ 3.0 at 6/12 months, or whether the biological/smi used at baseline was still used after 6/12 months. Proportions were compared using a Chi-square or Fisher’s exact test where appropriate. Non-parametrical data were compared using a Mann-Whitney U test.
p < 0.05 was considered statistically significant. All analyses were performed in SPSS Statistics software, version 25.0 (IBM, Armonk, NY, USA).
Results
Patient and disease characteristics
shows the patient characteristics (n = 194). Mean age of patients was 52 years (SD 13), and 79/189 was female (42%). A majority was in a relationship (132/186, 71%), and almost all had secondary or higher education (182/191, 95%). Mean disease duration was 19 years (IQR 11-35 years), and one in three patients had concomitant PsA (53/185, 29%). Most patients had low to intermediate comorbidity scores (low 84/197, 43%; intermediate 85/194, 44%; high 25/194, 13%). Dispersion of patient data throughout time points, including explanation of missing data, is shown in .
Figure 1. Inclusion of patients and explanation of missing data.
Patients were included if they had filled out a PRODISQ questionnaire at baseline and at least 1 follow-up timepoint (i.e. 6 or 12 months). 102 patients provided data for all three timepoints, 67 patients provided data on baseline and 6 months only, and 25 patients provided data on baseline and 12 months only.
All patients provided data on their work-for-pay (WFP) status (inclusion criteria). Only patients with WFP could provide information on presenteeism and overall work impairment. Not all patients filled in the TIC-P questionnaire, and therefore not all patients provided data on impairment in activities of daily living (ADL). Only patients with a filled in TICP, who were taking care of underage children, could provide data about child care.
N/A = not applicable; WFP = work-for-pay.
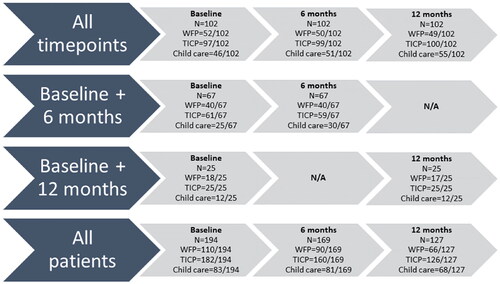
Table 1. Sample characteristics at baseline.
Disease characteristics and health status during 12 months follow-up
shows the follow-up data of the cohort, where timepoint differences were tested using GEE with the different timepoints as independent variables. At M12, the number of patients using the same biological/smi as at baseline had dropped significantly (M6 159/169 − 94%, M12 99/127 − 78%, p < 0.001). Both objective skin disease activity, as well as skin-specific QoL, improved in comparison to baseline (PASI: baseline 11.2 ± 7.2; M6 3.9 ± 4.6, p < 0.001; M12 3.9 ± 4.0, p < 0.001; DLQI: baseline 4, IQR 1-10; M6 1, IQR 0-4, p < 0.001; M12 2, IQR 2-5, p < 0.001). Moreover, also general physical and mental functioning improved significantly (PCS: baseline 43.6 ± 10.2; M6 46.1 ± 10.3, p < 0.001; M12 45.4 ± 11.0, p = 0.01; MCS: baseline 48.1 ± 11.4; M6 50.1 ± 10.8, p = 0.01; 12 months 51.0 ± 10.0, p = 0.01).
Table 2. Disease characteristics, work and ADL impairment at baseline and during follow-up.
Work-for-pay and work impairment during 12 months follow-up
shows the course of work-related parameters over a 12-month period, again using GEE with the different timepoints as independent variables to test for differences between timepoints. At baseline, 110/94 (57%) had work-for-pay. When comparing the baseline percentage of work-for-pay between the study population to the general Dutch population, the study population showed a lower employment rate than expected (work-for-pay BioCAPTURE 53% versus general population 67%, χ2 test, p = 0.01). The percentage of patients with work-for-pay did not change during follow-up (M6 53%, p = 0.09; M12 52%, p = 0.13).
Regarding work impairment, absenteeism was low throughout the entire follow-up (baseline 0% of maximum work hours, IQR 0-5; M6 0%, IQR 0-0, p = 0.01; M12 0%, IQR 0-5, p = 0.76), whereas presenteeism showed a statistically significant improvement at 12 months, but not at 6 months (baseline 5% of maximum theoretical productivity, IQR 0-18; M6 0%, IQR 0-15, p = 0.17; M12 0%, IQR 0-10, p = 0.04). Overall work impairment showed improvement over time, which was significant at 6 months but not 12 months (baseline 14%, IQR 0-26; M6 months 3%, IQR 0-20, p = 0.01; M12 2%, IQR 0-23, p = 0.49).
Associations between work impairment and disease-related characteristics/contextual factors
shows the results of the GEE, exploring relationships for work impairment with disease-related characteristics and contextual factors. In a logistic GEE model, being in a relationship (OR 2.12, 95%CI 1.04-4.33, p = 0.04) and remaining on the same biological/smi (OR 3.22, 95%CI 1.00-10.39, p = 0.05) were positively associated with the likelihood of having work-for-pay. However, female sex (OR 0.48, 95%CI 0.25-0.93, p = 0.03), a higher age (OR 0.89, 95%CI 0.86-0.92, p < 0.001), and a higher amount of comorbidity (low vs high OR 0.22, 95%CI 0.07-0.67, p = 0.01) were negatively associated with the likelihood of having work-for-pay. Disease activity and QoL parameters showed no significant relationship with work-for-pay status.
Table 3. Associations between work-for-pay status, presenteeism, impairments in ADL, and disease-related characteristics/contextual factors.
Next, we explored relationships for presenteeism (a quantitative marker of work impairment) with disease-related characteristics and contextual factors using a linear GEE model. Remaining on the same biological/smi (B = 13.20, 95%CI 2.52, 23.89, p = 0.02) and a higher amount of comorbidity (low vs intermediate B = 5.75, OR 1.04-10.46, p = 0.02) showed a positive association with a higher presenteeism (more impairment at work). Skin-related QoL (DLQI: B = 0.42, 95%CI 0.06-0.79, p = 0.02), and physical and mental functioning (PCS: B= −0.64, 95% CI −0.87 to −0.41, p < 0.001; MCS: B= −0.57, 95% CI −0.78 to −0.37, p < 0.001) showed a negative association with a higher presenteeism. In other words, deterioration of skin-related QoL by 1 point is associated with an increase in presenteeism of 0.4%, on a population level.
ADL impairment during 12 months follow-up
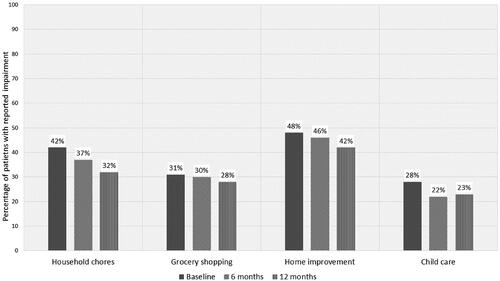
and show the baseline and follow-up data of the ADL-related parameters, using GEE with the different timepoints as independent variables to test for differences between timepoints. A substantial part of patients reported impairment in their ADL at baseline, of which home maintenance was most affected (impairment in household chores 37%; impairment in grocery shopping 31%; impairment in home maintenance 48%; impairment in childcare 28%). None of the ADL impairments changed during follow-up.
Associations between ADL impairment and disease-related characteristics/contextual factors
shows the results of the GEE, exploring relationships for ADL impairment with disease-related characteristics and contextual factors. Being in a relationship showed a negative relation with being impaired in household chores (OR 0.40, 95%CI 0.18-0.87, p = 0.02). Disease activity showed a negative association with being impaired in household chores (OR 0.95, 95%CI 0.91-1.00, p = 0.05) and being impaired in home maintenance (OR 0.94, 95%CI 0.89-0.99, p = 0.02). A higher amount of comorbidity showed a positive association with being impaired in grocery shopping (low vs intermediate OR 3.95, 95%CI 1.70-9.17, p = 0.001). Physical and mental functioning showed a negative association with being impaired in all ADL domains (e.g. household chores: PCS OR 0.85, 95%CI 0.82-0.89, p < 0.001; MCS OR 0.94, 95%CI 0.91-0.97; p < 0.001).
Association between treatment success and work/ADL impairment
Supplemental Table 1 shows the percentage of patients with work/ADL impairment, split per timepoint. Comparisons were made between patients with and without treatment success, where treatment success was defined as PASI ≤ 1.0, PASI ≤ 3.0, or retainment of the same biological/smi as used at baseline. Reaching PASI ≤ 1.0 after 12 months of treatment was associated with higher likelihood of having work-for-pay. Having treatment success was not associated with any of the outcomes on ADL impairment.
Discussion
Using prospective, longitudinal data from the BioCAPTURE cohort, we show that Dutch patients with plaque psoriasis who use biologicals/smi are less likely to have work-for-pay than the general Dutch population. Those who had work-for-pay reported a low percentage of overall work impairment, and this improved further over a 12 month period. Work-for-pay status was related to demographic variables (i.e. sex, age, and relationship status), while presenteeism was related to retainment of the first biological/smi, comorbidity, and mental/physical functioning. Moreover, up to half of patients report impairments in ADL. Improvement of objective disease activity was associated with improvement in ADL impairments. However, despite treatment success, the percentage of patients who experience impairments in ADL did not improve in the first year.
Regarding work-for-pay, patients with psoriasis were less likely to have paid employment than the Dutch general population. Although in this study we did not ask for the reason for not having work-for-pay, a survey in the United States showed that 92% of patients with psoriasis who did not have work-for-pay reported that having psoriasis was the main reason for their unemployment (Citation4). Interestingly, patients with longstanding PsA are also less likely to have work-for-pay than the general population, while this is not the case for patients with early PsA (Citation30, Citation31). Note that patients in the BioCAPTURE cohort had a disease duration of 19 years on average, before initiating the biological. Hypothetically, as in PsA, it could also be the case that patients with long-standing psoriasis are less likely to have work-for-pay than patients with early disease, i.e. that patients with Pso become unemployed during their disease. In the future, the possible relationship between disease duration and employment deserves future exploration in a psoriasis cohort with less longstanding disease to see if loss of work-for-pay arises during the disease, and to see if effective treatment could be protective against loss of work-for-pay.
In patients who have work-for-pay, we found an overall work impairment of 14% at baseline. This is comparable to other observational psoriasis cohort studies (Citation8, Citation32–35), while interventional studies with psoriasis patients report a higher level of overall work impairment up to 34% (Citation15, Citation18, Citation20, Citation36). This discrepancy between observational and interventional studies may be explained by a difference in the studied populations. In interventional studies, patients with a more pronounced disease are usually selected to ascertain that the intervention can achieve a beneficial effect; while in observational studies a more representative cross-selection of all patients is studied. Thus, interventional studies usually select patients with worse disease status, who presumably might have more work impairment. Indeed, previous studies have shown that a higher disease activity is associated with more work impairment (Citation34, Citation37–39).
During follow-up, we saw an improvement in both presenteeism and overall work impairment after treatment, which is in line with other interventional studies (Citation14, Citation15, Citation18–21, Citation36, Citation40). Although we found no association of presenteeism over-time with disease activity over-time, several studies did report that a larger treatment effect (e.g. a larger decrease in disease activity) was associated with more improvement in work impairment (Citation9, Citation16, Citation17), while another study found no significant correlation (Citation14). This difference may be partly explained by group size, differences in study setting (clinical trial versus registry), or by differences between countries (Citation32). In conclusion, the relationship between presenteeism and disease activity needs further exploration.
Up to half of the patients in our study reported impairment in ADL. This is in line with other international cohorts (Citation14, Citation33, Citation41). During 12 month follow-up, we found no change in the percentage of patients who felt impaired in ADL over time. However, other studies do report a decrease in the “amount” of impairment in ADL per person (Citation14, Citation15, Citation19–21, Citation36). We did observe a significant positive relationship between disease activity and ADL impairment. Tentatively, this suggests that while ADL impairment can improve after treatment, a significant number of patients do not reach a disease status in which they feel no ADL impairments at all.
Limitations of our study are the missing data in the registry, and the dichotomous way in which we measured ADL impairments. Perhaps, a more sensitive scale (i.e. Likert-scale, visual analogue scale or numerical rating scale) would have revealed differences in ADL impairments between baseline and follow-up. Moreover, our BioCAPTURE registry only contains patients with moderate-to-severe psoriasis treated with biologicals/smi, which may hamper external validity in patients with less severe psoriasis.
Strengths of our study are the exploration of different aspects of ADL impairment, identifying home maintenance as one of the most affected areas. Moreover, our study is the first to report changes in work impairment in patients with psoriasis after treatment with biologicals/smi in a non-trial, real-world setting. This setting may make our results more transferable to daily clinical practice.
In conclusion, our BioCAPTURE registry data revealed that Dutch psoriasis patients who are treated with biologicals/smi are less likely to have work-for-pay than the general population. During one year of treatment with biologicals/smi, we saw improvements in presenteeism and overall work impairment. Moreover, we saw a significant relationship between less disease activity and less ADL impairment, suggesting that effective treatment has a positive influence on the daily life of patients. Since patients state that one of their main treatment goals is “to experience less influence of psoriasis on daily activities, such as working, studying or sports” (Citation12), future research should be aimed at unraveling what causes these perceived impairments, with the ultimate goal to diminish them. We suggest that mapping out work and ADL impairments in a cohort with shorter disease duration would be a good starting point for this exploration, where a possible early intervention might have a protective effect against these impairments.
Ethics approval and informed consent
Under Dutch law, this non-interventional study is exempt from ethics review by the medical ethical committee. Informed consent was obtained from all patients before inclusion in the study.
Supplemental Material
Download PDF (718 KB)Acknowledgements
The authors would like to thank Elke ter Haar and Sarah Thomas for their help with the comorbidity scores.
Disclosure statement
TvH received personal fees from Eli Lily and Novartis, and non-financial support from UCB, outside the submitted work. JvdR carried out clinical trials for AbbVie, Celgene and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, LEO Pharma and Eli Lilly and reimbursement for attending a symposium from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University Medical Center Nijmegen, the Netherlands. MO has acted as consultant for Eli Lilly. MT has carried out clinical trials for Abbvie, Amgen, Novartis, Eli Lilly, Leo Pharma, Cellgene. All trial funding is not personal but goes to the independent research fund of the department of dermatology of Bravis Hospital Bergen op Zoom, the Netherlands. He has received speaking fees/attended advisory boards from Novartis, UCB and Pfizer and reimbursement for attending a symposium from UCB. SD has attended advisory boards for Abbvie, Janssen and Leo Pharma, and has received a congress fee from Abbvie. MvD has received consulting fees or honorarium from Novartis, Abbvie, Pfizer, Leopharma, Sanofi, Lilly, Janssen and Celgene, has received a grant and payment for lectures including service on speakers bureaus from Novartis, Sanofi and Janssen outside the submitted work. JJM attended an advisory board for Novartis. RT has attended advisory boards from Leo Pharma and Eli Lilly Netherlands.
PvL has received funding from Wyeth for research; carried out clinical trials for Abbott, Janssen; has received speaking and consulting fees from Wyeth, Schering-Plough; has received reimbursement for attending a symposium from Schering-Plough, Pfizer; has attended advisory boards for Abbvie, Leo Pharma, Novartis and UCB. JV had received speaker fee from Galapagos Netherland b.v. outside the submitted work. EdJ has received research grants for the independent research fund of the department of dermatology of the Radboud university medical center Nijmegen, the Netherlands from AbbVie, BMS, Janssen Pharmaceutica, Leo Pharma, Lilly, Novartis, and UCB for research on psoriasis and has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis or eczema including AbbVie, Amgen, Almirall, Celgene, Galapagos, Janssen Pharmaceutica, Lilly, Novartis, Leo Pharma, Sanofi and UCB. All funding is not personal but goes to the independent research fund of the department of dermatology of Radboud University medical center Nijmegen, the Netherlands. All other authors have no conflicts of interest to declare.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Augustin M, Radtke MA. Quality of life in psoriasis patients. Expert Rev Pharmacoecon Outcomes Res. 2014;14(4):1–9. doi: 10.1586/14737167.2014.914437.
- Raval K, Lofland JH, Waters H, et al. Disease and treatment burden of psoriasis: examining the impact of biologics. J Drugs Dermatol. 2011;10(2):189–196.
- Sumpton D, Kelly A, Tunnicliffe DJ, et al. Patients’ perspectives and experience of psoriasis and psoriatic arthritis: a systematic review and thematic synthesis of qualitative studies. Arthritis Care Res (Hoboken). 2020;72(5):711–722.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the national psoriasis foundation survey data 2003-2011. PLoS One. 2012;7(12):e52935. doi: 10.1371/journal.pone.0052935.
- Rasmussen MK, Enger M, Dahlborn AK, et al. The importance of achieving clear or almost clear skin for patients: results from the nordic countries of the global. Acta Derm Venereol. 2019;99(2):158–163. doi: 10.2340/00015555-3048.
- Orbai AM, Reddy SM, Dennis N, et al. Work absenteeism and disability associated with psoriasis and psoriatic arthritis in the USA-A retrospective study of claims data from 2009 TO 2020. Clin Rheumatol. 2021;40(12):4933–4942. doi: 10.1007/s10067-021-05839-9.
- Strober B, Greenberg JD, Karki C, et al. Impact of psoriasis severity on patient-reported clinical symptoms, health-related quality of life and work productivity among US patients: real-world data from the corrona psoriasis registry. BMJ Open. 2019;9(4):e027535. doi: 10.1136/bmjopen-2018-027535.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey. J Eur Acad Dermatol Venereol. 2018;32(10):1702–1709. doi: 10.1111/jdv.14986.
- Lebwohl M, Soliman AM, Yang H, et al. Impact of PASI response on work productivity and the effect of risankizumab on indirect costs using machine learning in patients with moderate-to-severe psoriasis. J Dermatolog Treat. 2022;33(4):2094–2101.
- Reich K, Foley P, Han C, et al. Guselkumab improves work productivity in patients with moderate-to-severe psoriasis with or without depression and anxiety: results from the VOYAGE 2 comparator study versus adalimumab. J Dermatolog Treat. 2020;31(6):617–623. doi: 10.1080/09546634.2019.1628172.
- Wu JJ, Lin C, Sun L, et al. Minimal clinically important difference (MCID) for work productivity and activity impairment (WPAI) questionnaire in psoriasis patients. J Eur Acad Dermatol Venereol. 2019;33(2):318–324. doi: 10.1111/jdv.15098.
- Kouwenhoven TA, van der Ploeg JAM, van de Kerkhof PCM. Treatment goals in psoriasis from a patient perspective: a qualitative study. J Dermatolog Treat. 2020;31(1):13–17. doi: 10.1080/09546634.2018.1544408.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16(8):981–990. doi: 10.1111/ddg.13613.
- Saeki H, Kanai Y, Murotani K, et al. Work productivity in real-life employed patients with plaque psoriasis: results from the ProLOGUE study. J Dermatol. 2022;49(10):970–978. doi: 10.1111/1346-8138.16517.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatolog Treat. 2016;27(6):552–555. doi: 10.3109/09546634.2016.1165339.
- Warren RB, Halliday A, Graham CN, et al. Secukinumab significantly reduces psoriasis-related work impairment and indirect costs compared with ustekinumab and etanercept in the United Kingdom. J Eur Acad Dermatol Venereol. 2018;32(12):2178–2184. doi: 10.1111/jdv.15094.
- Feldman SR, Zhao Y, Gilloteau I, et al. Higher psoriasis skin clearance is associated with lower annual indirect costs in the United States: a post hoc analysis from the CLEAR study. J Manag Care Spec Pharm. 2018;24(7):617–622. doi: 10.18553/jmcp.2018.24.7.617.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152(6):661–669. doi: 10.1001/jamadermatol.2016.0269.
- Revicki D, Willian MK, Saurat JH, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2008;158(3):549–557. doi: 10.1111/j.1365-2133.2007.08236.x.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66(2):e67-76–e76. doi: 10.1016/j.jaad.2010.10.020.
- Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28(6):790–798. doi: 10.1111/jdv.12177.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8.
- Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x.
- Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002.
- Koopmanschap MA. PRODISQ: a modular questionnaire on productivity and disease for economic evaluation studies. Expert Rev Pharmacoecon Outcomes Res. 2005;5(1):23–28. doi: 10.1586/14737167.5.1.23.
- Bouwmans C, De Jong K, Timman R, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv Res. 2013;13(1):217. doi: 10.1186/1472-6963-13-217.
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. doi: 10.2307/2531734.
- Statistiek CBvd. De arbeidsmarkt in cijfers 2019. In: statistiek CBvd, editor. Den Haag/Heerlen/Bonaire; 2020.
- van Hal TW, Mulder MLM, Wenink MH, et al. Determinants of work and social participation in patients with psoriatic arthritis in The Netherlands: an observational study. BMC Rheumatol. 2022;6(1):49. doi: 10.1186/s41927-022-00279-7.
- Wervers K, Luime JJ, Tchetverikov I, et al. Time to minimal disease activity in relation to quality of life, productivity, and radiographic damage 1 year after diagnosis in psoriatic arthritis. Arthritis Res Ther. 2019;21(1):25. doi: 10.1186/s13075-019-1811-4.
- Villacorta R, Teeple A, Lee S, et al. A multinational assessment of work-related productivity loss and indirect costs from a survey of patients with psoriasis. Br J Dermatol. 2020;183(3):548–558. doi: 10.1111/bjd.18798.
- Bronckers I, van Geel MJ, van de Kerkhof PCM, et al. A cross-sectional study in young adults with psoriasis: potential determining factors in quality of life, life course and work productivity. J Dermatolog Treat. 2019;30(3):208–215. doi: 10.1080/09546634.2018.1506077.
- Schaefer CP, Cappelleri JC, Cheng R, et al. Health care resource use, productivity, and costs among patients with moderate to severe plaque psoriasis in the United States. J Am Acad Dermatol. 2015;73(4):585–593 e3. doi: 10.1016/j.jaad.2015.06.049.
- Chan B, Hales B, Shear N, et al. Work-related lost productivity and its economic impact on Canadian patients with moderate to severe psoriasis. J Cutan Med Surg. 2009;13(4):192–197. doi: 10.2310/7750.2009.08068.
- Vender R, Lynde C, Ho V, et al. Work productivity and healthcare resource utilization outcomes for patients on etanercept for moderate-to-severe plaque psoriasis: results from a 1-year, multicentre, open-label, single-arm study in a clinical setting. Appl Health Econ Health Policy. 2012;10(5):343–353. doi: 10.1007/BF03261868.
- Kimball AB, Luger T, Gottlieb A, et al. Long-term impact of ixekizumab on psoriasis itch severity: results from a phase III clinical trial and long-term extension. Acta Derm Venereol. 2018;98(1):98–102. doi: 10.2340/00015555-2801.
- Hayashi M, Saeki H, Ito T, et al. Impact of disease severity on work productivity and activity impairment in Japanese patients with psoriasis. J Dermatol Sci. 2013;72(2):188–191. doi: 10.1016/j.jdermsci.2013.06.003.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41(5):514–521. doi: 10.1111/ced.12841.
- Strober B, Patil D, McLean RR, et al. Impact of secukinumab on patient-reported outcomes in biologic-naive patients with psoriasis in a US real-world setting. J Dermatolog Treat. 2022;33(8):3178–3187.
- Lewis-Beck C, Abouzaid S, Xie L, et al. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer Adherence. 2013;7:199–205. doi: 10.2147/PPA.S39887.