Abstract
Background: Injection site reaction (ISR) is a local phenomenon defined as a constellation of symptoms, including swelling, erythema, pruritus, and pain around the site of injection.
Objective: ISR is reported as a frequent adverse event after subcutaneous injection (SCI) of several biologics.
Methods: We performed an observational real-life study to compare dupilumab and tralokinumab as regards ISR, analysing frequency, duration and intensity of symptoms related to SCI. From January 2023 to June 2023, we enrolled adult patients affected by moderate to severe AD and being on dupilumab or tralokinumab treatment. A 12 items questionnaire was administered to all enrolled patients.
Results and conclusions: Three hundred and ninety-two patients were included. ISR was a frequent occurrence in both the treatment groups, with tralokinumab causing ISR more frequently than dupilumab. However, the reactions were generally mild and no patient stopped therapy.
Keywords:
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease clinically characterized by chronic or recurrent dermatitis and intense itching (Citation1, Citation2). It has a great impact on the mental health of patients and accounts for the major burden of global skin diseases (Citation1, Citation2). The etiology of AD covers a multitude of factors, like the impaired skin barrier function, unbalanced immunity, genetic susceptibility, and environmental exposure (Citation3, Citation4).
Progress in the understanding of the immunological mechanisms of AD has led to the recognition of multiple novel therapeutic targets (Citation5).
The approval of biologic drugs for moderate-severe forms has been crucial to the management of this disease. Dupilumab was the first biologic drug approved for moderate-to-severe AD from 6 months of age. The drug binds the alpha subunit of the interleukin (IL)-4 receptor and therefore blocks IL-4 and IL-13 signaling pathways (Citation6). The other approved drug is tralokinumab that binds IL-13 (Citation7). Both the biologics are administered by subcutaneous injection (SCI). Unlike intramuscular or intravenous injections, SCI does not require the intervention of skilled personnel, facilitates patient self-administration in home or outpatient clinical environments, provides a reduction in medical facility fixed costs, and provides flexibility in the anatomical infusion site (Citation8). The most common cutaneous adverse event of SCI of biological agents is injection site reaction (ISR), which is defined as a constellation of symptoms, including swelling, erythema, pruritus, and pain around the site of injection (Citation9).
Materials and methods
We performed an observational real-life study to compare dupilumab and tralokinumab as regards ISR, analyzing frequency, duration, and intensity of symptoms related to SCI. From January 2023 to June 2023, we enrolled adult (≥ 18 years) patients attending the Dermatology Unit of the University of Naples Federico II affected by moderate-to-severe AD and on dupilumab or tralokinumab treatment. A 12 items questionnaire was administered to patients (). The questionnaire was conceived and designed by the Authors. It was divided into 2 parts: the first consisted of 6 questions and examined the patient’s data such as age and sex and clinical history (atopic comorbidities, duration of treatment, prior biologic therapies); the second part included other 6 items evaluating whether the patient had experienced swelling, pain, burning, or erythema after the injection, and the duration of these symptoms. Numerical Rating Scale (NRS) (range 0-10) was used to quantitively evaluate pain/burning experienced. Reactions were always confirmed by evaluation by a physician.
The influence of ISR on delay or interruption in treatment was also asked. The questionnaire was completed by the patient anonymously and independently after the visit.
Quantitative variables were expressed as the mean and standard deviation (SD). Qualitative variables were expressed as frequencies and percentages. Graph Pad Prom software (v 8.0; Graph Pad software Inc. La Jolla, CA, USA) was used for all statistical analyses. The Mann-Whitney test, Fisher test and chi square test were used as appropriate to calculate statistical differences; a value of p ≤ 0.05 was considered significant.
Results
Three hundred and ninety-two patients were included in our study, 214 males (54.59%) and 178 females (45.41%) with a mean age of 50.2 years ±17.6. Among these patients, 327 (83.42%) received dupilumab and 65 (16.58%) tralokinumab, respectively; this data is influenced by the more recent market release for AD of tralokinumab. All the patients were bio-naive. At baseline, 91/392 (23.21%) patients had a family history of AD; 115/392 (29.33%) reported at least 1 concurrent atopic comorbidity, with allergic rhinitis accounting for 18.11% (71/392), asthma for 9.69% (38/392), and conjunctivitis for 7.14% (28/392) of cases ().
Table 1. Baseline patient characteristics.
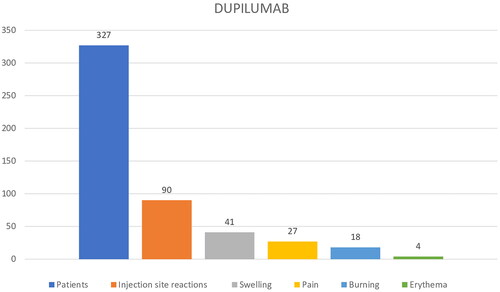
Among dupilumab patients, 90/327 (27.52%) reported ISR: 41/90 (45.5%) patients experienced swelling, 27/90 (30%) pain, 18/90 (20%) burning, and 4/90 erythema (4.45%) (). The mean NRS value for pain/burning was of 4.9 ± 1.6.
ISR firstly appeared during the first or second month of treatment in 12/90 (13.34%) and 36/90 (40%) patients, respectively; he remaining 42/90 (46.67%) patients showed ISR after the third month. The mean duration of ISR varied between few minutes to a maximum of 2 days with gradual decrease in frequency and severity with continuation of injections.
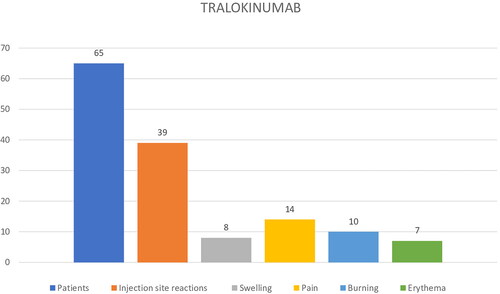
Conversely, 39/65 (60%) tralokinumab treated patients reported ISR: 35.89% (14/39) experienced pain, 25.65% (10/39) burning, 20,51% swelling (8/39), and 17.94% (7/39) erythema, respectively (). The mean pain/burning NRS value reported was of 7.2 ± 2.3. ISR onset occurred in 33.84% (22.65) of patients during the first month of tralokinumab therapy, and in 38/65 (58.46%) during the second month. Regarding the duration, ISR commonly occurred within few minutes and persisted until 3-5 days after tralokinumab injections; the same frequency and severity of ISR with continuation of injections was recorded. A statistically significant difference was found between the prevalence of ISR (p < 0.05) in the dupilumab group compared to the tralokinumab group (p < 0.05). No patients discontinued therapy due to ISR in both the groups. ().
Table 2. Results ISR and NRS value for both groups.
ISR was managed with short courses of topical corticosteroid therapy (mometasone furoate) in 35/90 (38.89%) patients of dupilumab group, and in 22/39 (56.41%) of tralokinumab group, whit resolution in a few days.
Discussion
The signs and symptoms related to ISR are often underestimated, even if they can impact the patients’ adherence to the treatment and consequently the clinical outcomes (Citation10, Citation11). Dupilumab safety data were predominantly derived from 12 randomized, placebo-controlled trials, including AD, asthma, and chronic rhinosinusitis with nasal polyposis patients. In all these studies, a common adverse reaction (≥ 1/100 to < 1/10) was ISR (Citation12). In AD patients ISR was reported from 12.3% of patients treated with dupilumab compared to 7.2% of patient treated with placebo (Citation13). ISR was of mild or moderate severity in all the cases, self-limiting, and required no treatment or dupilumab discontinuation (Citation13). For tralokinumab, from a pool of 5 randomized, double-blind, placebo-controlled studies in patients with moderate to severe AD, ISR occurred more frequently in patients who received tralokinumab (7.2%) compared to placebo (3.0%) (Citation14–16), other reports of ISR have been reported in recent real-life studies (Citation17, Citation18).
The vast majority (99%) of ISRs was mild or moderate in severity, and few patients (< 1%) discontinued tralokinumab treatment (Citation14–16).
In our real-life setting, we recorded a higher percentage of ISR in tralokinumab group compared to dupilumab group (28% vs 60%). However, the two groups of patients were numerically quite different (327 patients in dupilumab group vs 65 patients in tralokinumab group). As regard clinical symptoms of ISR, the most frequent were swelling and burning in dupilumab group, while pain and burning in tralokinumab group. Pain/burning intensity was higher in patients treated with tralokinumab (7.2 ± 2.3 vs 4.9 ± 1.6 mean pain-NRS). Recently, De Greef A et al. reported in a multicentric prospective study a prevalence of 19% of ISR between 21 AD patients treated with tralokinumab (Citation16). In 2 cases tralokinumab was stopped for important and invalidating skin reactions at injection site (Citation16).
ISR can be classified into 2 groups according to the pathogenic mechanism: irritative reactions (immediate) and allergic reactions (immediate or delayed) to the excipient or to the drug itself (Citation9, Citation15).
A pre-filled syringe of dupilumab contains l-arginine monohydrochloride, l-histidine, l-histidine monohydrochloride monohydrate, polysorbate 80, sodium acetate trihydrate, acetic acid glacial, sucrose, and water for injections; tralokinumab solution contains sodium acetate trihydrate, acetic acid, sodium chloride, polysorbate 80, and water for injections (Citation8, Citation12). According to literature data, it is possible that polysorbate 80 may be a significant factor in IRS (Citation19, Citation20). Sensitization to polysorbate 80 may occur during therapy or it may preexist in susceptible individuals because these (and other nonionic surfactants) are widely used, including in cosmetic and food products (Citation19, Citation20).
Finally, it is possible that the higher frequency of ISR in tralokinumab group may be due to the higher frequency of administrations compared to dupilumab (4 vs 2 at baseline and 2 vs 1 every 2 weeks).
Conclusion
The limitation of our study relates to the difference in sampling of patients treated with dupilumab compared to patients treated with tralokinumab, this certainly does not allow direct comparison but may open new future study scenarios to test our data on the frequency of ISR. This difference is undoubtedly influenced by the more recent marketing of tralokinumab than dupilumab. Furthermore, in patients with ISR we didn’t perform patch test with polysorbate 80
On the other hand, the strength of our study is that it is the first reported real-life experience of these 2 drugs regarding ISR. No patient discontinued treatment, even among those in the tralokinumab group. In these patients one possibility to reduce reactions could be dose spacing of the drug.
Currently, there is lack of evidence on how to approach ISR. Patient education and counseling prior to the initiation of the treatment is important to avoid unnecessary early discontinuation or scarce adherence of the medication. The physician should inform the patient on the possibility of developing ISR, especially during the first months of treatment and encourage them to continue the treatment.
Informed consent
The patients in this manuscript have given written informed consent to publication of their case details.
All authors read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Ständer S. Atopic dermatitis. N Engl J Med. 2021;384(12):1–5. doi: 10.1056/NEJMra2023911.
- Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. 2021;22(8):4130. doi: 10.3390/ijms22084130.
- Napolitano M, Fabbrocini G, Martora F, et al. Role of aryl hydrocarbon receptor activation in inflammatory chronic skin diseases. Cells. 2021;10(12):3559. doi: 10.3390/cells10123559.
- Mandlik DS, Mandlik SK. Atopic dermatitis: new insight into the etiology, pathogenesis, diagnosis and novel treatment strategies. Immunopharmacol Immunotoxicol. 2021;43(2):105–125. doi: 10.1080/08923973.2021.1889583.
- Wollenberg A, Werfel T, Ring J, et al. Atopic dermatitis in children and adults—diagnosis and treatment. Dtsch Arztebl Int. 2023;120(13):224–234. doi: 10.3238/arztebl.m2023.0011.
- Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–282. doi: 10.1016/S0140-6736(22)01199-0.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi: 10.1111/bjd.19574.
- https://www.prnewswire.com/news-releases/subcutaneous-biologics-technologies-and-drug-delivery-systems-2nd-edition-2018-2030-300696232.html. (last access 2 August 2023)
- Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Ther. 2019;32(2):e12817. doi: 10.1111/dth.12817.
- Gast A, Mathes T. Medication adherence influencing factors-an (updated) overview of systematic reviews. Syst Rev. 2019;8(1):112. doi: 10.1186/s13643-019-1014-8.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease - strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12):2253–2263. doi: 10.1111/jdv.15913.
- https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf. (last access 2 August 2023).
- Thaçi D, L Simpson E, Deleuran M, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci. 2019;94(2):266–275. doi: 10.1016/j.jdermsci.2019.02.002.
- https://www.ema.europa.eu/en/documents/product-information/adtralza-epar-product-information_en.pdf. (last access 2 August 2023).
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450–463. doi: 10.1111/bjd.19573.
- De Greef A, Ghislain PD, Bulinckx A, et al. Real-Life experience of tralokinumab for the treatment of adult patients with severe atopic dermatitis: a multicentric prospective study. Clin Drug Investig. 2023;43(4):299–306. doi: 10.1007/s40261-023-01258-7.
- Pezzolo E, Schena D, Gambardella A, et al. Survival, efficacy and safety of tralokinumab after 32 and 52 weeks of treatment for moderate-to-severe atopic dermatitis in adults: a multicentre real-world study. J Eur Acad Dermatol Venereol. 2024;38(1):e11–e13. doi: 10.1111/jdv.19382.
- Gargiulo L, Ibba L, Vignoli CA, et al. Tralokinumab rapidly improves subjective symptoms and quality of life in patients with moderate-to-severe atopic dermatitis: a real-life 16-week experience. J Dermatolog Treat. 2023;34(1):2216815. doi: 10.1080/09546634.2023.2216815.
- Corominas M, Gastaminza G, Lobera T. Hypersensitivity reactions to biological drugs. J Investig Allergol Clin Immunol. 2014;24(4):212–225; quiz 1p following 225.
- Singh SK, Mahler HC, Hartman C, et al. Are injection site reactions in monoclonal antibody therapies caused by polysorbate excipient degradants? J Pharm Sci. 2018;107(11):2735–2741. doi: 10.1016/j.xphs.2018.07.016.