To the Editor,
Alopecia areata (AA) continues to be a real therapeutic challenge. However, the unmet need for therapeutic options and the increasing understanding of intracellular pathways involved in skin inflammation has led to the development of JAK-inhibitors (JAKi) emerging as potential therapies for AA (Citation1). Baricitinib, a JAK1/2 inhibitor, was recently approved by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) for the treatment of AA.
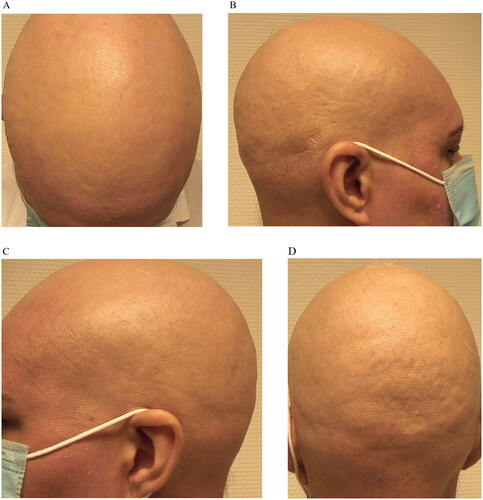
A 45-year-old woman presented with a 9-year history of alopecia areata and atopic dermatitis and no report of other interest comorbidities presented to our hospital for evaluation of a new relapse. Physical examination showed hair loss distributed by eyebrows, occipital and parietal region with a Severity of Alopecia Tool (SALT) score of 95 and with an Eczema Area Severity Index (EASI) score of 24, Scoring Atopic Dermatitis (SCORAD) of 37, Patient Oriented Eczema Measure (POEMS) of 16 and Dermatology Life Quality Index (DLQI) 14 of for her.
She had been previously treated with systemic corticosteroids (Prednisone 0.5 mg/kg/day two to three times a year in a decreasing regimen for 3 years), cyclosporine (3.5 mg/kg/day for 1 year), mycophenolate mofetil (500 mg of every 8 h for 12 months) and topical potent steroids (0.5 mg/g clobetasol propionate in cream) without clinical benefit.
Then Baricitinib 4 mg/daily was prescribed with partial response regarding her AD (EASI 7) and also in AA (SALT 80) after four months of treatment, with a relapse in both pathologies after six months (EASI 26; SCORAD 41; POEMS 19; DLQI 18; SALT 98) ().
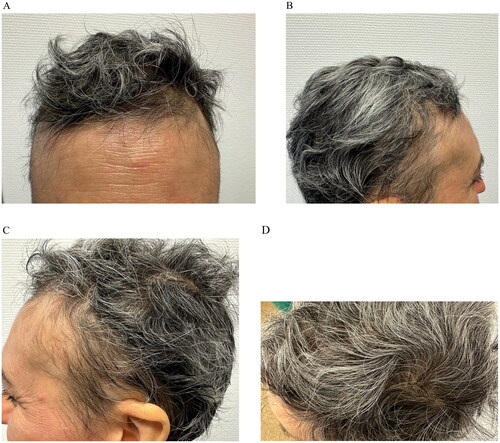
The lack of response to previous treatments led us to prescribe upadacitinib at the dosage of 30 mg/daily. Improvements in both AA and AD were noted after initiating upadacitinib and by 5 months treatment she had completed resolution of her AD (EASI 0; SCORAD 0; POEMS 0; DLQI 0) and AA (SALT 0) and no adverse events were detected after 12 months of treatment with Upadacitinib ().
The pathogenesis of atopic dermatitis (AD) and alopecia are both characterized by a modified expression of interleukins (IL) that belong to the T-helper type 2 inflammation’s pool and by genetic mutations of proteins involved in atopy and IgE-mediated immune response with a key role of IFN- γ and other upregulated cytokines (i.e., IL-2 and IL-15) which signal through JAK-1 (Citation2,Citation3). Upadacitinib is an oral, reversible and selective Janus kinase (JAK)-1 inhibitor recently been approved by EMA for the treatment of moderate-to-severe AD. There are some reports in the literature on the successful treatment of AA which reported the complete resolution with Upadacitinib 30 mg/daily (Citation2,Citation4–6).
Mostly, the primary indication for treatment was atopic dermatitis. Chiricozzi et al. presented a retrospective study of 19 patients with AD and AA with a greater improvement in SALT score, was detected in patients with high serum IgE levels ([100 kU/L) and/or with personal history of noncutaneous atopic comorbid conditions (Citation7). Asfour et al. reported a case report of a patient initially responded to treatment with Baricitinib for both AD and AA who had to withdraw the treatment after six weeks because of side effects including severe acne requiring systemic therapy, migraines, recurrent orolabial herpes simplex virus, and lethargy. Subsequently treated with Upadacitinib was achieved with great response (Citation8).
Focus on patients with partial response to baricitinib we presented evidence of the second case report successfully treated with upadacitinib with completed resolution. The first one reported showed a therapeutic switch due to the lack of response to baricitinib after nine months of treatment, which raises the possibility of a cumulative effect, potentiated by the previous inhibition of baricitinib in the JAK-STAT pathway (considering a recent study revealing the efficacy of baricitinib in patients with severe adult AA continued to improve over 52 weeks) (Citation5,Citation9). Otherwise, in our case, we assessed the response after the patient experienced a relapse of his pathology while being treated with baricitinib, which supports the importance of selective JAK1 inhibition to improve alopecia areata (Citation5) and suggests the possible efficacy of upadacitinib after loss of response to baricitinib.
A phase 3 study of Upadacitinib (NCT06012240) to Evaluate the Safety and Effectiveness of Upadacitinib Tablets in Adult and Adolescent Participants With Severe Alopecia Areata is actually being performed (Citation10).
To sum up, our case provides further evidence for the use of upadacitinib in the management of concurrent AA/AD, especially in the context of chronic cases such as ours who have previously shown partial response to baricitinib.
Consent form
The authors confirm written informed consent from the patients for the use of image and publication of their case details has been given, regarding their privacy.
Author contributions
Contributed significantly to this publication: Francisco Javier De la Torre Gomar, Juan Pablo Velasco Amador, Álvaro Prados Carmona and Ricardo Ruíz Villaverde.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Flora A, Kozera E, Frew JW. Treatment of alopecia areata with the janus kinase inhibitor upadacitinib: a retrospective cohort study. J Am Acad Dermatol. 2023;89(1):1–3. doi: 10.1016/j.jaad.2022.12.056.
- Gori N, Cappilli S, Di Stefani A, et al. Assessment of alopecia areata universalis successfully treated with upadacitinib. Int J Dermatol. 2023;62(2):e61–e63. doi: 10.1111/ijd.16342.
- Novielli D, Foti C, Principi M, et al. Upadacitinib in concurrent Crohn’s disease, atopic dermatitis and alopecia areata: a case report. J Eur Acad Dermatol Venereol. 2023;38(1):e8–e10. doi: 10.1111/jdv.19377.
- Johnston LA, Lu C, Poelman SM. Successful treatment of concomitant alopecia universalis and Crohn’s disease with upadacitinib: a case report. SAGE Open Med Case Rep. 2023;11:2050313X231160914.
- Uchida H, Kamata M, Watanabe A, et al. Improvement of severe alopecia areata after 9-month baricitinib treatment followed by subsequent use of upadacitinib in a patient with atopic dermatitis. J Dermatol. 2023;50:e392–e393.
- Youssef S, Bordone LA. Effective treatment of alopecia universalis with oral upadacitinib. JAAD Case Rep. 2023;31:80–82. doi: 10.1016/j.jdcr.2022.08.014.
- Chiricozzi A, Balato A, Fabbrocini G, et al. Beneficial effects of upadacitinib on alopecia areata associated with atopic dermatitis: a multicenter retrospective study. J Am Acad Dermatol. 2023;89(6):1251–1253.
- Asfour L, Getsos Colla T, Moussa A, et al. Concurrent chronic alopecia areata and severe atopic dermatitis successfully treated with upadacitinib. Int J Dermatol. 2022;61(11):e416–e417. doi: 10.1111/ijd.16316.
- Kwon O, Senna MM, Sinclair R, et al. Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol. 2023;24(3):443–451. doi: 10.1007/s40257-023-00764-w.
- AbbVie. A phase 3 randomized, placebo-controlled, double-blind program to evaluate efficacy and safety of upadacitinib in adult and adolescent subjects with severe alopecia areata [Internet]. 2023 Dec [cited 2023 Jan 1]. Report No.: NCT06012240. Available from: https://clinicaltrials.gov/study/NCT06012240