Abstract
Purpose: Mast cells, their serine proteinase tryptase, and immunoglobulin E (IgE) can be involved in cutaneous carcinogenesis.
Materials and methods: To study the association of tryptase+ and IgE+ cells with photodamage and skin cancers 385 adult patients (201 males, 184 females, 75 with immunosuppression) at risk of any type of skin cancer were examined. Skin biopsies were taken from the sun-protected medial arm and from the photodamaged dorsal forearm skin followed by immunohistochemical staining for tryptase and IgE.
Results: The results show that tryptase+ and IgE+ cells are significantly higher in number in the photodamaged than sun-protected skin, both in immunocompetent and -compromised subjects, and there is a strong correlation between tryptase+ and IgE+ cells. The numbers of forearm tryptase+ and especially IgE+ cells associated significantly with the forearm photodamage severity. In the logistic regression analysis, the forearm to upper arm ratio of IgE+ cells produced a univariate odds ratio of 1.521 (p = .010) and a multivariate one of 3.875 (p = .047) for the history of squamous cell carcinoma. The serum level of total IgE correlated significantly to the IgE to tryptase ratio in both skin sites.
Conclusions: Therefore, IgE+ mast cells participate in photodamage and carcinogenesis, though it is unclear whether they are tumor-protective or -causative.
Introduction
Ultraviolet (UV) radiation from the sun is the major factor for the development of cutaneous photodamage and cancers (Citation1). The photodamage appears as dryness, irregular pigmentation, wrinkling, elastosis, and telangiectasia (Citation2). Even though previous research suggests that UVB causes most of the photocarcinogenesis, UVA is also involved in skin cancer development. UVA produces reactive oxygen species that damage membrane lipids with resultant activation of UV response genes (Citation2–4), as well as epidermal hyperplasia, stratum corneum thickening, Langerhans cell depletion, and accumulation of inflammatory cell infiltrates (Citation4).
Cutaneous mast cells can release a variety of mediators upon activation, including proteolytic enzymes, histamine, lipid-derived mediators, cytokines, chemokines, and growth factors (Citation5). Mast cells can be divided into two subgroups by their proteolytic enzymes: MCT cells contain only tryptase, but MCTC cells, the predominant cell type in the skin, contain tryptase, chymase, carboxypeptidase and cathepsin G (Citation6,Citation7). Mast cells can be involved in skin carcinogenesis through participation in immunosuppression, neovascularization, degradation of extracellular matrix (ECM), and tumor cell mitosis (Citation8). It has previously been suggested that mast cells may adopt either a proinflammatory or immunosuppressive phenotype depending on the cutaneous microenvironment (Citation9) and therefore the outcome may be either promotion or inhibition of tumor growth (Citation10). Mast cells may have a marked role in UVB-induced immunosuppression through different pathways. UVB induces isomerization of photo-receptor trans-urocanic acid to cis-form (Citation11) resulting in consequent neuropeptide secretion and mast cell degranulation. In addition, UVB affects keratinocytes to secrete nerve growth factor, which maintains the release of neuropeptides (Citation12). Mast cells secrete histamine and TNF-α that take part in the UVB-induced immunosuppression cascade (Citation13). In fact, many factors have been noticed to affect mast cell function after UV exposure, including endothelin-1, cis-urocanic acid, complement factor B, and platelet activating factor (Citation14).
Beta-tryptase, a tetrameric trypsin-like serine proteinase, is the major proteolytic enzyme that is secreted from mast cells upon degranulation (Citation15). Previously, it has been found that tryptase can degrade ECM by activating matrix metalloproteinases and by direct degradation, including fibronectin (Citation5,Citation16). The powerful chymotrypsin-like serine proteinase, chymase, can enhance these destructive changes if left without control by protease inhibitors (Citation17). The ECM damage leads to the destruction of basement membrane and photoaging. It has been reported previously that tryptase can have a significant role in collagen degradation (Citation18). In a previous study on the sun-protected and sun-exposed skin in preauricular area, tryptase+ cells were noticed to be more numerous in the sun-exposed than sun-protected skin (Citation19). Recently, an association between serum tryptase level and cutaneous photodamage and skin malignancy was observed (Citation20).
Immunoglobulin E (IgE) mediates immediate-type allergic reactions and plays a part in the defense against parasites and toxins. IgE binds to two different receptors, the high-affinity FcɛRI and low-affinity FcɛRII. FcɛRI is expressed in mast cells and basophils, but also in other immune cell types, including dendritic cells and eosinophils (Citation21–23). After allergen exposure, the cross-linking of antigen-specific IgE molecules on mast cells induces degranulation and liberation of preformed and newly-generated mediators. In previous studies, the relationship between IgE, atopy and cancer risk has been found to be conflicting, though IgE may function in tumor suppression (Citation21).
The correlation of serum IgE level to cutaneous photodamage, skin cancers, moles, and actinic keratosis has been studied recently, but significant associations were not observed (Citation24). A higher level of IgE has been associated with a higher risk for squamous cell carcinoma (SCC) (Citation25). Also, IgE has been found to be a part of the host defense against epithelial damage and tumor development after topical exposure to a DNA-damaging chemical, which suggests that IgE is tumor-protective (Citation26). A study on the malignancy risk in adults with an undetectable level of IgE (<3 IU/ml) revealed that these subjects have increased risk for a first malignancy, particularly hematologic one, compared to those with normal IgE level (Citation27). In a review, it was presented that the deficiency of IgE was connected to more rapid tumor growth and higher risk for any malignancy, especially in subjects with low serum and tissue IgE levels (Citation28).
In order to investigate the link between tryptase+ mast cells or IgE+ cells and photocarcinogenesis, skin biopsies were taken from 385 adult subjects with an elevated risk for any type of skin cancer. The biopsies were taken from both the sun-protected medial arm and sun-exposed dorsal forearm skin followed by immunohistochemical staining for tryptase and IgE. The immunostained cells were correlated to a variety of skin-related parameters, such as photodamage, actinic keratoses (AKs), pigment cell nevi and skin cancer history. In addition, the study subjects were divided to atopic and non-atopic subjects as well as to immunocompetent and -compromised subjects.
Materials and methods
Study subjects and skin biopsies
The study subjects (N = 385, aged 21–79) consisted of patients at the Dermatologic outpatient clinic in Kuopio University Hospital, Kuopio, Finland, as described (Citation20,Citation24). The entry criteria for participation in the study were the age of 18–80 years and an increased risk for any type of skin cancer. The subjects were recruited between May 2017 and October 2020, except for mid-summer months, June, and July. The study subjects filled out a questionnaire regarding, e.g., previous sun exposure, sunburns, UV-light treatments, indoor tanning, skin cancers, tobacco and alcohol usage, immunosuppression, and medications. The skin cancer risk was evaluated by experienced dermatologists, and the assessment was based on, e.g., past or present skin cancers or AKs, skin photodamage level, abundance of moles, atypical moles, immunosuppression, skin phototype, and family history of melanoma. The evaluation of immunosuppression was based on a use of immunosuppressive medication because of OTR or immune-mediated disease during the past several years at least three months per year, as described in detail previously (Citation20). After entry, the subjects were divided into a low, moderate, or high skin cancer risk class as described previously (Citation20,Citation24,Citation29). The atopic status was evaluated, and all subjects were divided into a non-atopy, mucous membrane atopy or skin atopy groups (Citation24). The non-atopy group consisted of 240 subjects. There were 79 subjects with mucous membrane atopy and 53 subjects with skin atopy alone or together with mucous membrane atopy (Citation24). All voluntarily attending subjects read an informative material and signed a written consent before entering the study. The study has been approved by the Ethics Committee of Kuopio University Hospital (71/2017) and followed the principles of the declaration of Helsinki.
A history of past malignancy in extracutaneous site (ECS) was verified in 52 subjects, including a cancer in breast, lung, prostate, liver, kidneys, bladder, intestine, pancreas, brain, hematologic, tongue, reproductive organs, salivary gland, tonsils, eye, or thyroid gland. With regard to a past or present history of skin cancers, the number of subjects with any skin cancer history was 220, comprising 75 with melanoma (both malignant (N = 63) and in situ (N = 12) types of melanomas), 155 with basal cell carcinoma (BCC), and 36 with cutaneous SCC (N = 30) or Bowen’s disease (N = 6) (Citation20,Citation24).
Skin biopsies were taken using a 4-mm punch tool under local anesthesia from the dorsal aspect of forearm skin (photodamaged skin) and from the medial aspect of upper arm skin (sun-protected skin). All biopsies were fixed in 10% formalin and then embedded in paraffin.
Immunohistochemical staining
A rabbit polyclonal antibody against human IgE was purchased from Thermo Fisher Scientific (Catalog number PA5-16396, MA, USA). The rabbit polyclonal antibody against purified human skin tryptase is an in-house antibody produced previously (Citation30).
The skin samples were processed for 5-µm sections followed by fixation in 10% formalin and immunohistochemical staining using a 1:700 dilution of anti-IgE or 0.183 µg/ml anti-tryptase antibody. The immunopositive cells were visualized using a Vectastain Elite ABC Rabbit IgG Kit (Vector PK-6101, Vector Laboratories, CA, USA). The cells immunopositive for IgE or tryptase were counted in separate sections from an area of 1.0 mm (width) × 0.6 mm (depth) immediately beneath the epidermis by using an ocular grid (Citation31,Citation32). All samples were analyzed with Leica DM 4000B light microscope equipped with a 40x Plan Leica objective.
The blood samples were taken from the cubital fossa vein from 381 subjects. Blood tests included complete blood cell count, serum tryptase and serum total IgE. IgE was analyzed with electrochemiluminescence immunoassay and serum tryptase with ImmunoCAPTM assay (Citation20,Citation24).
Statistics
Statistical analyses were performed with IBM SPSS Data Editor. The chi-square test was used to analyze categorical variables, and the unpaired, two-tailed, t-test continuous variables. The Fisher’s exact test was used in variables, which contained groups with fewer than five members. The analysis of variance (ANOVA) was used in analyses, which contained more than two groups. In the correlation analysis, the Spearman correlation test was used. The binary logistic regression analysis was used to assess the factors that may have an effect on the forearm photodamage level. A p value less than .05 was considered as statistically significant.
Results
Tryptase+ and IgE+ cells in the photodamaged versus sun-protected skin
The numbers of tryptase+ and IgE+ cells were significantly higher in the photodamaged than sun-protected skin, and the result was similar regardless of the immune status. Furthermore, there were strong correlations (p < .001) between tryptase+ and IgE+ cells in all cases (). Representative micrographs of the immunostainings are shown in .
Figure 1. Immunohistochemical staining for (A) mast cell tryptase and (B) IgE on skin sections from the forearm sun-exposed skin. Note that there are several IgE+ cells with cell membrane-like circular staining (B). The micrographs were taken using a 40× objective.
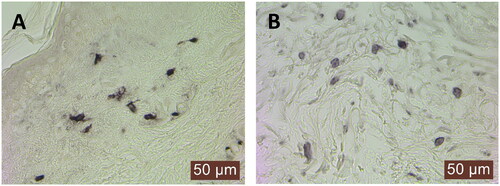
Table 1. The numbers of tryptase+ and IgE+ cells in the photodamaged forearm and sun-protected upper arm skin.
Correlation between immunopositive cells and different variables
To assess the upregulation of tryptase+ or IgE+ cells in the photodamaged skin, the ratio of cell numbers in the forearm to upper arm skin was calculated (). In the case of tryptase+ cells, no correlation was seen between this ratio and a variety of variables. The ratio of IgE+ cells correlated significantly to the facial photoaging score (p = .041) and age (p = .045). A borderline significance was seen in monocyte count (p = .052).
Table 2. Spearman correlation between the forearm/upper arm ratio of tryptase+ and IgE+ cells and different variables.
The number of tryptase+ cells in the forearm skin correlated to BMI (p = .033), and a borderline significance was seen in the case of skin tumor count (p = .051) (). Regarding other variables, no correlations were observed. The number of IgE+ cells in the forearm skin correlated to the forearm photoaging score (p = .025) and skin tumor count (p = .049).
Immunopositive cells in subjects with skin cancer history versus controls
The ratio of tryptase+ cells in the forearm to upper arm skin or the number of tryptase+ cells in the forearm skin did not differ significantly between the subjects with a history of any skin cancer, BCC, SCC, melanoma (all cases) or malignant melanoma and those without skin cancer history (Supplementary Table 1). In addition to skin cancers, malignancies in ECS or those in the lymphatic system were analyzed (the data of ECS malignancies are from (Citation24)), but there was no difference between the groups.
The ratio of IgE+ cells and the number of IgE+ cells in the forearm skin were compared between these groups, too (Supplementary Table 1). The ratio of IgE+ cells was higher in the subjects with malignancy in ECS than in those without it (p = .016). In addition, the subjects with SCC history revealed a higher ratio of IgE+ cells compared to those without SCC (p = .051). However, the number of IgE+ cells in the forearm skin revealed no significant differences in any of these subgroups.
The numbers of IgE+ and tryptase+ cells were analyzed also in the sun-protected skin. However, significant differences were not observed in any of the subgroups.
Logistic regression analyses
In the case of malignancy in ECS (Supplementary Table 2), the age equal to or above the median 66 produced a univariate OR 6.389 (p < .001), the moderate skin cancer risk class an OR 2.235 (p = .035), and the forearm/upper arm IgE ratio an OR 1.418 (p = .019).
With regard to the history of SCC (Supplementary Table 3), significant univariate ORs were seen in age (OR 16.320, p < .001), gender (female, OR 0.335, p = .006), lifetime sun exposure (very often, OR 5.659, p = .011), smoking history (OR 2.662, p = .010), skin cancer risk class (moderate risk, OR 10.605, p = .023; high risk, OR 38.000, p < .001) and forearm/upper arm IgE ratio (OR 1.521, p = .010). In multivariate analysis, significances were seen in age (p < .001), lifetime sun exposure (occasionally, p = .044; very often, p = .002), skin cancer risk class (high risk group, p = .004) and, again, IgE ratio (OR 3.875, p = .047).
In the analysis of subjects with a photodamage score 2–4 compared to control subjects with a score 0–1 in the forearm skin (), female subjects showed a univariate OR 0.665 compared to male subjects (p = .048), the indoor work showed an OR 0.382 compared to outdoor work (p = .020), smoking showed an OR 1.685 (p = .012), and the advanced age revealed an OR 7.139 (p < .001). The high skin cancer risk class produced an OR 3.203 (p < .001) and the moderate risk class an OR 1.800 (p = .013) compared to low-risk group. The number of tryptase+ or IgE+ cells in the forearm skin showed a significant univariate OR of 1.009 (p = .024) or 1.014 (p = .007), respectively. BMI, immunosuppression, indoor tanning, sunburns, lifetime sun exposure, tryptase+ or IgE+ cell counts in the upper arm skin, the ratio of IgE to tryptase in the forearm skin, tryptase or IgE ratios between the forearm and upper arm skin, did not show association with the severity of forearm photodamage. In the multivariate analysis, only the age showed significance with an OR 7.051 (p < .001). In the case of other variables, significant ORs were not reached.
Table 3. The logistic regression analysis and consequent odds ratios for subjects with a photodamage score 2–4 (N = 208) compared to control subjects with a score 0–1 (N = 176) in the forearm skin in all subjects.
Comparisons of immunopositive cells in atopic and non-atopic subjects
In all and immunocompetent subjects, significant differences were observed in the number of tryptase+ and IgE+ cells in atopy and non-atopy groups (). In both groups, tryptase+ and IgE+ cells were in a similar fashion higher in the photodamaged than sun-protected skin. In all subjects, the cell count of forearm tryptase+ cells was higher in the non-atopy than atopy group (p = .034).
Table 4. Comparison of tryptase+ and IgE+ cells between atopic and non-atopic subjects.
In immunocompromised subjects (), the numbers of tryptase+ and IgE+ cells were higher in the forearm than upper arm skin in the non-atopy group, but not in the atopy group. With regard to the ratio of IgE to tryptase, no significant differences were seen.
Comparisons of immunopositive cells between three atopy groups
When both atopy groups and non-atopy group were compared to each other, significant differences were not observed in the number of tryptase+ or IgE+ cells in either forearm or upper arm skin (Supplementary Table 4). However, a significantly higher ratio of IgE to tryptase was seen in the sun-protected skin in the MM atopy group when compared to that in the non-atopy group (p = .012).
Correlation between IgE or tryptase in serum and immunopositive cells in skin
IgE in serum correlated positively to IgE+ cells in the sun-protected skin (p = .041, N = 147) (Supplementary Table 5). Also, the ratio of IgE to tryptase in the upper arm and forearm skin correlated positively to serum IgE (p < .001). However, serum tryptase (N = 294) did not reveal any significant correlation to IgE+ cells, tryptase+ cells or the ratio between them in either skin sites.
Subjects with a very low serum immunoglobulin E
In subjects with a measured serum total IgE, 9 subjects out of 147 (6.1%) revealed a serum level lower than or equal to 2.5 kU/l. Three of these subjects were immunocompromised and six were immunocompetent. One subject had history of melanoma, one a history of BCC, and one a history of both BCC and SCC. Three subjects had a history of atopy. Cancer in ECS (breast cancer) was in two subjects. Despite very low serum IgE, IgE+ cells were detected in all 9 subjects in the forearm (range 13.3–46.7 cells/mm2, mean ± SD 29.4 ± 10.2) and upper arm skin (range 11.7–43.3 cells/mm2, 27.0 ± 9.3), and these cell numbers did not differ significantly from the cell numbers of other 138 subjects (forearm 36.0 ± 18.1 cells/mm2 and upper arm 30.1 ± 14.4 cells/mm2).
Discussion
In this study, tryptase+ and IgE+ cells were significantly higher in the photodamaged than sun-protected skin, and there was a strong correlation between these cells regardless of the immune status. The forearm tryptase+ and especially IgE+ cells associated with the forearm photodamage severity. In addition, the forearm to upper arm ratio of IgE+ cells produced significant univariate and multivariate ORs for the history of SCC. The serum level of total IgE correlated significantly to the IgE to tryptase ratio in both upper arm and forearm skin. The limitation of this study was that all study subjects filled out the questionnaires by themselves and for this reason, personal interpretation of questions may affect the results. The strength is that all subjects were examined by experienced dermatologists.
In previous studies, mast cell tryptase has been found to activate matrix metalloproteinases (Citation33), induce the proliferation of endothelial cells (Citation34), and increase the proliferation of fibroblasts and the synthesis of collagen type I (Citation35,Citation36). In addition, tryptase can cause focal epidermal-dermal separation and fibronectin degradation in the basement membrane ex vivo (Citation16). In this study, tryptase+ cells were significantly increased in the photodamaged skin, and in the univariate analysis they associated with photodamage severity, which supports the theory that tryptase is involved in photodamage processes. The result of the increase in tryptase+ cells is supported by the previous study showing that the number of cutaneous mast cells is higher in distal than proximal extremities (Citation37). Another factor, which could have an effect on the photodamage severity, besides tryptase, is the smoking history (Citation38), like it was found in the univariate analysis in this study. Interestingly, the number of tryptase+ cells has previously been found to be increased in the healthy and sun-protected skin of tobacco smokers compared to nonsmokers (Citation32). One possibility, how smoking may participate in these events, is the inactivation of α1-proteinase inhibitor, a known inhibitor of elastase and mast cell chymase (Citation39,Citation40). Nevertheless, because tryptase can have regenerative properties in ECM, in addition to degradative ones, the net effect of these bidirectional mechanisms can determine the final outcome.
IgE+ cells were increased in the photodamaged skin, as did tryptase+ cells. The past or present history of atopy may play some role in these cellular changes because of the higher ratio of IgE to tryptase in the sun-protected skin, but not in the photodamaged skin, in subjects with MM atopy compared to non-atopic subjects. This result parallels our recent result on the differences in serum IgE between these 3 different patient groups (Citation24). In addition, the status of immunosuppression mostly prevented these increases in IgE+ and tryptase+ cells in atopic, but not in non-atopic subjects. In comparison to tryptase+ cells, IgE+ cells showed a stronger positive association with the severity of forearm photodamage in both correlation and univariate regression analyses. The strong correlation between IgE+ and tryptase+ cells suggests that tryptase+ mast cells constitute the predominant cell type expressing IgE, though dermal dendritic cells may express IgE, too (Citation22). Previously, an experimental study found that a DNA damage in mouse skin induced by an environmental carcinogen initiates stress surveillance by γδTCR-positive intraepithelial lymphocytes, an autoreactive IgE response, and consequent protection against carcinogenesis. UV-irradiation was reported to induce an IgE response, too. Repeated exposure to the carcinogen led to the development of papillomas and SCCs as well as rising serum IgE followed by accumulation of IgE in acutely damaged skin and tumors, in which IgE bound mainly to FcεRI on basophils (Citation26). The dermal IgE identified in this study represents total IgE, not an antigen-specific one. The ratio of IgE to tryptase in the forearm and upper arm skin as well as IgE+ cells in the upper arm skin correlated significantly to the serum level of total IgE. It is not known whether this serum or dermal IgE is protumorigenic or antitumorigenic or whether it contains an IgE molecule that recognizes a specific antigen in the photodamaged skin. However, the results suggest that the more photodamage is caused by solar UV light the more extensive is the IgE response in the serum and damaged forearm skin. The age, male gender, skin cancer risk class, smoking and outdoor working history were found to be risk factors for the forearm photodamage, too, but this is expectable.
A higher number of intralesional tryptase+ cells has previously been connected to a better survival rate in deeply invasive melanomas and a less advanced stage in superficially invasive melanomas (Citation31). In the BCC lesion, tryptase+ mast cells are increased in number (Citation41). In SCC, the number of tryptase+ mast cells has been reported to be lower in higher grades of SCC, though the result was not statistically significant (Citation42). In addition, the lower expression of FcεRI+ cells correlated to more severe SCC disease (Citation26). Therefore, both tryptase+ and IgE+ cells were studied in their relation to the subjects with a past or present history of skin cancer. Nevertheless, there was no significant difference in these cell numbers or in the cellular ratio of forearm to arm skin with regard to any skin cancer, BCC, SCC or melanoma. Therefore, these cellular biomarkers in the photodamaged skin appear not to associate with or predict skin cancers in this cross-sectional study setting. The higher mast cell prevalence in the non-sun-exposed buttock skin has been connected to higher risk for BCC (Citation12). Significant difference in dermal mast cell count in the buttock skin was not observed when patients with a history of SCC were compared to healthy control subjects (Citation43). Like in the case of BCC, in patients with a history of melanoma, the buttock skin mast cell count was higher compared to control subjects (Citation44). In the present study, tryptase+ and IgE+ cell counts in the sun-protected skin did not differ significantly between the subjects with and without BCC, SCC, or melanoma.
The forearm/upper arm ratio of IgE+ cells was higher in subjects with a history of malignancy in ECS than in controls. A similar higher ratio was seen in the case of SCC history, but only with a borderline significance. In the logistic regression analysis of the malignancy in ECS, the ratio of IgE+ cells produced a significant univariate OR 1.418. In the case of SCC, the OR 1.521 by IgE ratio was significant, too. In the multivariate analysis on the malignancy in ECS, the IgE ratio was not significant, but in the case of SCC, the p-value remained significant with an even higher OR 3.875.
The association between serum IgE and cancer diagnosis has been studied previously. IgE of over 35 kU/l had an inverse association with cancer risk, but an effect on cancer survival was not seen (Citation45). The topical exposure to 7,12-dimethylbenz[a]anthracene has been noticed to induce a unique autoreactive IgE response and knockout mice without IgE response developed larger tumors more rapidly than mice with normal IgE function (Citation26). The studies by Weller et al. (Citation27) and Ferastraoaru et al. (Citation28) also support the hypothesis of tumor-protective effect of IgE. In this study, the dermal IgE may not just be a causal factor for carcinogenesis, such as photodamage and SCC, because it may also be interpreted to be an attempt for a tumor-protective reaction by IgE. On the other hand, the outcome can depend on the type of IgE, because carcinogen-induced autoreactive IgE showing unique repertoire with specific VDJ rearrangements and CDRH3 characteristics can be tumor-protective, whereas chronic inflammation can induce a polyclonal IgE response with natural specificity and repertoire that may promote carcinogenesis (Citation26,Citation46). Therefore, the blocking of IgE response in chronically inflamed skin might be beneficial to prevent carcinogenesis.
In conclusion, mast cells, tryptase and IgE are involved in skin photodamage and carcinogenesis toward the SCC line of lesions. However, it is unclear whether the dermal IgE is a causal factor for carcinogenesis or, in fact, is related to tumor-protective response. Future research should be focused on a possible antigen-specific IgE in carcinogenetic environment. In light with this aim, recent studies suggest that a tumor antigen-specific IgE can be utilized in cancer immunotherapy, including melanoma (Citation47–49).
Ethics statement
All study subjects signed an informed consent before entering the study. The study was approved by (71/2017) by the Ethics Committee of Kuopio University Hospital, Kuopio, Finland and followed the principles of the declaration of Helsinki.
Supplemental Material
Download Zip (71.5 KB)Acknowledgements
Ms Anne Koivisto and Ms Katja Dufva are acknowledged for expert technical assistance. Mr Tuomas Selander MSc is acknowledged for statistical advises.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The research data is available from the corresponding author upon separate request.
Additional information
Funding
References
- Leiter U, Keim U, Garbe K. Epidemiology of skin cancer: update 2019. Adv Exp Med Biol. 2020;1268:1–9. doi: 10.1007/978-3-030-46227-7_6.
- Matsumura Y, Ananthaswamy HN. Short-term and long-term cellular and molecular events following UV irradiation of skin: implications for molecular medicine. Expert Rev Mol Med. 2002;4(26):1–22. doi: 10.1017/S146239940200532X.
- Kochevar IE. Molecular and cellular effects of UV radiation relevant to chronic photodamage. In: Gilchrest BA, editor. Photodamage. New York: Blackwell Scientific Publishers; 1995. p. 51–58.
- Lavker RM, Gerberick GF, Veres D, et al. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J Am Acad Dermatol. 1995;32(1):53–62. doi: 10.1016/0190-9622(95)90184-1.
- Harvima IT, Nilsson G, Suttle M-M, et al. Is there a role for mast cells in psoriasis? Arch Dermatol Res. 2008;300(9):461–478. doi: 10.1007/s00403-008-0874-x.
- Schechter NM, Irani AM, Sprows JL, et al. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145(8):2652–2661. doi: 10.4049/jimmunol.145.8.2652.
- Irani AM, Bradford TR, Kepley CL, et al. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37(10):1509–1515. doi: 10.1177/37.10.2674273.
- Ch’ng S, Wallis RA, Yuan L, et al. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19(1):149–159. doi: 10.1038/modpathol.3800474.
- Harvima IT, Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta Derm Venereol. 2011;91(6):644–650. doi: 10.2340/00015555-1197.
- Ribatti D, Tamma R, Crivellato E. The dual role of mast cells in tumor fate. Cancer Lett. 2018;433(433):252–258. doi: 10.1016/j.canlet.2018.07.005.
- Wille JJ, Kydonieus AF, Murphy GF. Cis-urocanic acid induces mast cell degranulation and release of preformed TNF-alpha: a possible mechanism linking UVB and cis-urocanic acid to immunosuppression of contact hypersensitivity. Skin Pharmacol Appl Skin Physiol. 1999;12(1-2):18–27. doi: 10.1159/000029842.
- Grimbaldeston MA, Skov L, Finlay-Jones JJ, et al. Increased dermal mast cell prevalence and susceptibility to development of basal cell carcinoma in humans. Methods. 2002;28(1):90–96. doi: 10.1016/s1046-2023(02)00213-x.
- Hart PH, Grimbaldeston MA, Finlay-Jones JJ. Sunlight, immunosuppression, and skin cancer: role of histamine and mast cells. Clin Exp Pharmacol Physiol. 2001;28(1-2):1–8. doi: 10.1046/j.1440-1681.2001.03392.x.
- Siiskonen H, Smorodchenko A, Krause K, et al. Ultraviolet radiation and skin mast cells: effects, mechanisms and relevance for skin diseases. Exp Dermatol. 2018;27(1):3–8. doi: 10.1111/exd.13402.
- Harvima RJ, Harvima IT, Dull D, et al. Identification and characterization of multiple forms of tryptase from human mast cells. Arch Dermatol Res. 1999;291(2-3):73–80. doi: 10.1007/s004030050386.
- Kaminska R, Helisalmi P, Harvima RJ, et al. Focal dermal–epidermal separation and fibronectin cleavage in basement membrane by human mast cell tryptase. J Invest Dermatol. 1999;113(4):567–573. doi: 10.1046/j.1523-1747.1999.00738.x.
- Huttunen M, Harvima IT. Mast cell tryptase and chymase in chronic leg ulcers: chymase is potentially destructive to epithelium and is controlled by proteinase inhibitors. Br J Dermatol. 2005;152(6):1149–1160. doi: 10.1111/j.1365-2133.2005.06428.x.
- Iddamalgoda A, Le QT, Ito K, et al. Mast cell tryptase and photoaging: possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Arch Dermatol Res. 2008;300 Suppl 1(S1):S69–S76. doi: 10.1007/s00403-007-0806-1.
- Bosset S, Bonnet-Duquennoy M, Barré P, et al. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br J Dermatol. 2003;149(4):826–835. doi: 10.1046/j.1365-2133.2003.05456.x.
- Komulainen J, Siiskonen H, Harvima IT. Association of elevated serum tryptase with cutaneous photodamage and skin cancers. Int Arch Allergy Immunol. 2021;182(11):1135–1142. doi: 10.1159/000517287.
- Sutton BJ, Davies AM, Bax HJ, et al. IgE antibodies: from structure to function and clinical translation. Antibodies. 2019;8(1):19. doi: 10.3390/antib8010019.
- Bieber T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (Fc epsilon RI). Immunobiology. 2007;212(6):499–503. doi: 10.1016/j.imbio.2007.03.001.
- Barata LT, Ying S, Grant JA, et al. Allergen-induced recruitment of Fc&RI + eosinophils in human atopic skin. Eur J Immunol. 1997;27(5):1236–1241. doi: 10.1002/eji.1830270527.
- Komulainen J, Siiskonen H, Haimakainen S, et al. Patients with a history of atopy have fewer cutaneous melanomas and malignancies in extracutaneous sites than those without atopy: a study in 496 patients at risk of skin cancers. Melanoma Res. 2023;33(3):218–229. doi: 10.1097/CMR.0000000000000887.
- Wiemels JL, Wiencke JK, Li Z, et al. Risk of squamous cell carcinoma of the skin in relation to IgE: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2377–2383. doi: 10.1158/1055-9965.EPI-11-0668.
- Crawford G, Hayes MD, Seoane RC, et al. Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response. Nat Immunol. 2018;19(8):859–870. doi: 10.1038/s41590-018-0161-8.
- Weller KN, McDonnell JC, Albert JM, et al. Increased hazard risk of first malignancy in adults with undetectable serum IgE: a retrospective cohort study. J Clin Immunol. 2023;43(3):568–577. doi: 10.1007/s10875-022-01401-7.
- Ferastraoaru D, Jordakieva G, Jensen-Jarolim E. The other side of the coin: IgE deficiency, a susceptibility factor for malignancy occurrence. World Allergy Organ J. 2021;14(1):100505. doi: 10.1016/j.waojou.2020.100505.
- Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients–where do we stand today? Am J Transplant. 2008;8(11):2192–2198. doi: 10.1111/j.1600-6143.2008.02386.x.
- Harvima IT, Naukkarinen A, Harvima RJ, et al. Immunoperoxidase and enzymehistochemical demonstration of human skin tryptase in cutaneous mast cells in normal and mastocytoma skin. Arch Dermatol Res. 1988;280(6):363–370. doi: 10.1007/BF00426615.
- Siiskonen H, Poukka M, Bykachev A, et al. Low numbers of tryptase + and chymase + mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res. 2015;25(6):479–485. doi: 10.1097/CMR.0000000000000192.
- Kaukinen A, Fitzgibbon A, Oikarinen A, et al. Increased numbers of tryptase-positive mast cells in the healthy and sun-protected skin of tobacco smokers. Dermatology. 2014;229(4):353–358. doi: 10.1159/000365189.
- Gruber BL, Marchese MJ, Suzuki K, et al. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84(5):1657–1662. doi: 10.1172/JCI114344.
- Blair RJ, Meng H, Marchese MJ, et al. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99(11):2691–2700. doi: 10.1172/JCI119458.
- Abe M, Kurosawa M, Ishikawa O, et al. Mast cell tryptase stimulates both human dermal fibroblast proliferation and type I collagen production. Clin Exp Allergy. 1998;28(12):1509–1517. doi: 10.1046/j.1365-2222.1998.00360.x.
- Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;99(6):1313–1321. doi: 10.1172/JCI119290.
- Weber A, Knop J, Maurer M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol. 2003;148(2):224–228. doi: 10.1046/j.1365-2133.2003.05090.x.
- Ortiz A, Grando SA. Smoking and skin. Int J Dermatol. 2012;51(3):250–262. doi: 10.1111/j.1365-4632.2011.05205.x.
- Taggart C, Cervantes-Laurean D, Kim G, et al. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275(35):27258–27265. doi: 10.1074/jbc.M004850200.
- Schechter NM, Sprows JL, Schoenberger OL, et al. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989;264(35):21308–21315. doi: 10.1016/S0021-9258(19)30080-8.
- Diaconu N-C, Kaminska R, Naukkarinen A, et al. The increase in tryptase- and chymase-positive mast cells is associated with partial inactivation of chymase and increase in protease inhibitors in basal cell carcinoma. J Eur Acad Dermatol Venereol. 2007;21(7):908–915. doi: 10.1111/j.1468-3083.2006.02100.x.
- Biswas A, Richards JE, Massaro J, et al. Mast cells in cutaneous tumors: innocent bystander or maestro conductor? Int J Dermatol. 2014;53(7):806–811. doi: 10.1111/j.1365-4632.2012.05745.x.
- Grimbaldeston MA, Skov L, Finlay-Jones JJ, et al. Squamous cell carcinoma is not associated with high dermal mast cell prevalence in humans. J Invest Dermatol. 2002;119(5):1204–1206. doi: 10.1046/j.1523-1747.2002.19511.x.
- Grimbaldeston MA, Pearce AL, Robertson BO, et al. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br J Dermatol. 2004;150(5):895–903. doi: 10.1111/j.1365-2133.2004.05966.x.
- Wulaningsih W, Holmberg L, Garmo H, et al. Investigating the association between allergen-specific immunoglobulin E, cancer risk and survival. Oncoimmunology. 2016;5(6):e1154250. doi: 10.1080/2162402X.2016.1154250.
- Hayes M, Ward S, Crawford G, et al. Inflammation-induced IgE promotes epithelial hyperplasia and tumour growth. Elife. 2020;9:e51862. doi: 10.7554/eLife.51862.
- Chauhan J, Grandits M, Palhares LCGF, et al. Anti-cancer pro-inflammatory effects of an IgE antibody targeting the melanoma-associated antigen chondroitin sulfate proteoglycan 4. Nat Commun. 2023;14(1):2192. doi: 10.1038/s41467-023-37811-3.
- Spicer J, Basu B, Montes A, et al. Safety and anti-tumor activity of the IgE antibody MOv18 in patients with advanced solid tumors expressing folate receptor-alpha: a phase I trial. Nat Commun. 2023;14(1):4180. doi: 10.1038/s41467-023-39679-9.
- Nakamura M, Souri EA, Osborn G, et al. IgE activates monocytes from cancer patients to acquire a pro-inflammatory phenotype. Cancers. 2020;12(11):3376. doi: 10.3390/cancers12113376.

