Abstract
Background
Deucravacitinib is a selective oral tyrosine kinase 2 (TYK2) inhibitor recently approved for psoriasis.
Objectives
We aimed to evaluate the real-world effectiveness and safety of deucravacitinib for psoriasis.
Methods
We analyzed 33 Japanese patients with psoriasis (23 with plaque psoriasis, eight with psoriatic arthritis, and two with erythrodermic psoriasis) from January 2023 to October 2023. All patients received deucravacitinib 6 mg daily until week 16.
Results
At week 8, 12, or 16, the achievement rate of PASI 75 was 60.9%, 73.9%, or 78.3%, that of PASI 90 was 13.0%, 39.1%, or 52.2%, that of PASI 100 was 0%, 8.7%, or 13.0%, that of absolute PASI ≤2 was 34.8%, 65.2%, or 78.3%, respectively. The achievement rate of dermatology life quality index 0/1 at week 16 was 42.9%. Fourteen patients (42%) complained pruritus. Peak pruritus-numerical rating scale in patients with pruritus decreased by median [interquartile] 71.4 [50–80] % of baseline at week 2. Adverse events occurred in 18.2% of patients, which were mild and manageable.
Conclusions
Deucravacitinib for patients with psoriasis was well-tolerated and gave favorable therapeutic effects in the real-world practice. Deucravacitinib treatment rapidly reduced pruritus.
Introduction
Psoriasis is a chronic inflammatory skin disease with enhanced immune axis of interleukin (IL)-23/IL-17 (Citation1,Citation2). Tyrosine kinase 2 (TYK2) is a member of the Janus kinase (JAK) family, and mediates intracellular signalings from cytokines, type I interferons (IFNs) (IFN-α, β), IL-12, and IL-23 (Citation3).
Deucravacitinib selectively inhibits TYK2 by binding pseudokinase domain (an allosteric inhibition), and is an oral once daily medicine first developed as TYK2 inhibitor. Clinical trials have reported high efficacy and safety of deucravacitinib for psoriasis patients including those from Japan; however, data from real-world clinical practice remain limited (Citation4–6). Further, laboratory parameters reflecting the therapeutic effects of deucravacitinib on psoriasis are not established. To date, values of C-reactive protein (CRP), neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR), and platelet to lymphocyte ratio (PLR) are reported to reflect therapeutic effects of biologics (Citation7–12), and might also reflect those of deucravacitinib.
In this study, we aimed to evaluate the effectiveness and safety of deucravacitinib in real-world clinical practice. Since pruritus is known to be one of bothersome symptoms of psoriasis (Citation13), we also evaluated if deucravacitinib treatment may improve pruritus in psoriasis patients.
Methods
Study design and data collection
This study was conducted at the Department of Dermatology in Nippon Medical School Chiba Hokusoh Hospital. Between January and October 2023, 33 adult Japanese patients with psoriasis (23 with plaque psoriasis, eight with psoriatic arthritis, and two with erythrodermic psoriasis) who received deucravacitinib were enrolled. All patients were administered deucravacitinib 6 mg once daily until week 16 to analyze its effectiveness and safety. The diagnosis of psoriasis was made based on clinical findings.
Before treatment, we collected data on the patients’ age, sex, BMI, disease duration, previous treatment, presence or absence of arthritis, scalp, nail, or genital lesions, diabetes, and current smoking. Total (whole body) PASI and PASI on four individual anatomical sites, head and neck, trunk, upper limbs, and lower limbs were analyzed at weeks 0, 2, 4, 6, 8, 12, 14, and 16 of treatment. The values of NLR, MLR, PLR, and CRP were analyzed at weeks 0, 4, and 16. The values of serum immunoglobulin E (IgE) and total eosinophil count were evaluated before treatment. The achievement rates of PASI 75, 90, or 100 (the improvement of PASI from baseline ≥75%, ≥90%, or 100%, respectively), and of absolute PASI ≤2 or absolute PASI ≤1, and percent reduction of PASI were calculated at weeks 4, 8, 12, and 16. The patients reported their dermatology life quality index (DLQI), index of quality of life (QOL), at weeks 0 and 16. We examined the proportion of patients who achieved DLQI = 0 or 1 and reduction ≥2-point from baseline (DLQI 0/1). Patients reported peak pruritus-numerical rating scale (PP-NRS) for the assessment of pruritus. Among the 14 patients (42%) who reported PP-NRS >0 before treatment, all exhibited a baseline PP-NRS of 4 or higher. We examined the proportion of patients who achieved an improvement of ≥4-point from baseline PP-NRS among patients with baseline PP-NRS ≥4-point (PP-NRS 4) at week 2, 4, 8, 12, and 16. In this study, the therapeutic response was assessed using a modified Non-Responder Imputation (NRI) method.
Safety was assessed by the occurrence of treatment-emergent adverse events (TEAEs) during deucravacitinib treatment, until 30 days after the last dose of deucravacitinib. A TEAE was defined as any adverse event (AE) that began or worsened after the initiation of treatment. Patients provided written informed consent. Data were retrospectively analyzed using medical records.
Statistical analysis
All statistical analyses were performed using the EZR (Saitama Medical Center, Jichi Medical School, Saitama, Japan). Results are expressed as the mean ± standard deviation for variables with a normal distribution or as the median and interquartile range for variables with a non-parametric distribution. Differences between weeks 0, 2, 4, 6, 8, 10, 12 and 16 were analyzed using Friedman’s test with Bonferroni’s post hoc test. For paired comparisons between two related groups, Wilcoxon’s signed-rank test was used. The correlations of the variables with each other were analyzed by Spearman’s correlation coefficients. Statistical significance was set at p < .05.
Results
Demographics and baseline characteristics of patients treated with deucravacitinib
Thirty-three Japanese patients with psoriasis (24 males and nine females) were included in the study (). shows baseline (week 0) values of total PASI, PASI on four anatomical sites, DLQI and baseline values of laboratory parameters. Seven patients (21%) were pretreated systemically; four patients with biologics and three patients with apremilast. Pruritus was present in 14 patients (42.2%).
Table 1. Baseline characteristics of patients with psoriasis treated with deucravacitinib (n = 33).
The transition of total PASI and PASI on individual anatomical sites during treatment with deucravacitinib
Total PASI significantly decreased at week 2 by median [interquartile range] 25.4 [10.9–33.2] % of baseline, continued to decrease thereafter, and plateaued at week 8 with 80.4 [64.5–86.4] % reduction from baseline (). PASI on head and neck significantly decreased at week 4 by 40.0 [17.5–73.8] % of baseline, gradually decreased thereafter, and plateaued at week 8 with 90.8 [58.9–100.0] % reduction (). PASI on the trunk significantly decreased at week 6 by 66.7 [59.2–79.3] % of baseline, gradually decreased thereafter, and plateaued at week 10 with 100.0 [85.6–100.0] % reduction (). PASI on upper limbs significantly decreased at week 4 by 50.0 [20.0–62.5] % from baseline, gradually decreased thereafter, and plateaued at week 8 with 76.8 [66.7–90.2] % reduction (). PASI on lower limbs significantly decreased at week 4 by 50.0 [26.8–56.9] % of baseline, gradually decreased thereafter, and plateaued at week 10 with 81.3 [76.4–90.8] % reduction (). Thus, total PASI and PASI scores on four individual sites decreased mostly in parallel during treatment with deucravacitinib.
Figure 1. The transition of total psoriasis area and severity index (PASI) scores (a), PASI scores on head and neck (b), trunk (c), upper limbs (d), and lower limbs (e) during deucravacitinib treatment in patients with psoriasis (n = 33). Data are provided as the median [interquartile range]. *p < .05, **p < .01 versus values of week 0; †p < .05, ††p < .01 versus values of week 2; §p < .05 versus values of week 4; #p < .05 versus values of week 6, assessed by Friedman’s test with Bonferroni’s post hoc test.
![Figure 1. The transition of total psoriasis area and severity index (PASI) scores (a), PASI scores on head and neck (b), trunk (c), upper limbs (d), and lower limbs (e) during deucravacitinib treatment in patients with psoriasis (n = 33). Data are provided as the median [interquartile range]. *p < .05, **p < .01 versus values of week 0; †p < .05, ††p < .01 versus values of week 2; §p < .05 versus values of week 4; #p < .05 versus values of week 6, assessed by Friedman’s test with Bonferroni’s post hoc test.](/cms/asset/9b14877d-b888-4c3e-875c-1edd00780487/ijdt_a_2307489_f0001_c.jpg)
The changes of laboratory parameters after deucravacitinib treatment, and correlations between percent reductions of those parameters versus that of total PASI
The values of NLR, MLR, PLR, and CRP at weeks 4 and 16 were not significantly different from those of week 0 though NLR, MLR, or PLR appeared to decrease slightly (). The percent reductions of NLR, MLR, and PLR at week 4 were positively correlated with that of total PASI ().
Table 2. The laboratory parameters at weeks 0, 4, and 16 of deucravacitinib treatment, and the comparisons between the stages (n = 33).
Table 3. Correlations between percent reduction of total PASI versus those of laboratory parameters at weeks 4 or 16 of deucravacitinib treatment in patients with psoriasis (n = 33).
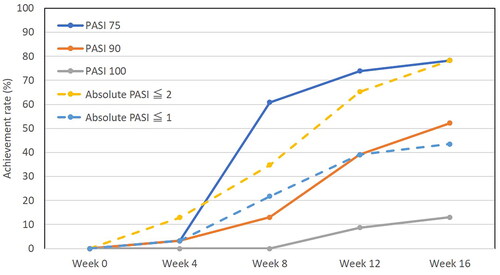
The achievement rates of PASI 75, PASI 90, PASI 100, absolute PASI ≤2, absolute PASI ≤1, and DLQI 0/1 during treatment with deucravacitinib
We assessed the achievement rates of PASI 75, PASI 90, PASI 100, absolute PASI ≤2, and absolute PASI ≤1 in patients with psoriasis during deucravacitinib treatment. At week 8, 12, or 16, the achievement rate of PASI 75 was 60.9%, 73.9%, or 78.3%, that of PASI 90 was 13.0%, 39.1%, or 52.2%, that of PASI 100 was 0%, 8.7%, or 13.0%, that of absolute PASI ≤2 was 34.8%, 65.2%, or 78.3%, and that of absolute PASI ≤1 was 21.7%, 39.1%, or 43.5%, respectively (). The DLQI at week 16 (median [interquartile range] 2 (1–4)) was significantly reduced compared to week 0 (10.5 (7–25)) (p < .01), by Wilcoxon’s signed-rank test), and achievement rate of DLQI 0/1 at week 16 was 42.9%.
The transition of PP-NRS and the achievement rates of PP-NRS 4 during deucravacitinib treatment in psoriasis patients with baseline pruritus
Fourteen patients (42.4%) complained of pruritus (PP-NRS >0) before treatment with deucravacitinib. We then evaluated if deucravacitinib treatment might reduce PP-NRS in the patients with pruritus. PP-NRS rapidly and significantly reduced at week 2 by median [interquartile] 71.4 [50–80] % of baseline, and plateaued thereafter (). The achievement rate of PP-NRS 4 was peaked at week 2 (64.3%) and mostly plateaued thereafter ().
Figure 3. The transition of peak pruritus-numerical rating scale (PP-NRS) (a), and achievement rate of PP-NRS4 (b) during deucravacitinib treatment in psoriasis patients with baseline pruritus (n = 14). Data are provided as the median [interquartile range]. *p < .05 versus values of week 0, assessed by Friedman’s test with Bonferroni’s post hoc test.
![Figure 3. The transition of peak pruritus-numerical rating scale (PP-NRS) (a), and achievement rate of PP-NRS4 (b) during deucravacitinib treatment in psoriasis patients with baseline pruritus (n = 14). Data are provided as the median [interquartile range]. *p < .05 versus values of week 0, assessed by Friedman’s test with Bonferroni’s post hoc test.](/cms/asset/643b57a4-fec7-4dbd-aa01-df8ff044db53/ijdt_a_2307489_f0003_c.jpg)
Correlations between percent reductions of PP-NRS versus those of clinical indexes at weeks 4, 8, 12, or 16 of deucravacitinib treatment in patients with baseline pruritus
We then analyzed if the percent reduction of PP-NRS in response to deucravacitinib might be correlated with those of clinical indexes (). The percent reduction of PP-NRS at week 4 was correlated with those of total PASI, PASI on trunk, upper, and lower limbs. The percent reduction of PP-NRS at week 12 was correlated with those of total PASI, PASI on head and neck, and trunk. The percent reduction of PP-NRS at week 16 was correlated with those of PASI on lower limbs and DLQI. The results indicate that the improvement of PP-NRS might at least partially correlate with that of other clinical indexes.
Table 4. Correlations between percent reductions of PP-NRS versus those of clinical indexes at weeks 4, 8, 12, or 16 of deucravacitinib treatment in psoriasis patients with baseline pruritus (n = 14).
Safety outcomes
The safety profile of deucravacitinib in this real-world practice study was mostly comparable to that in previous clinical trials (Citation4–6) (). No new safety concerns were identified for deucravacitinib. Adverse events occurred in six patients (18.2%). There were no severe AEs or AEs leading to death.
Table 5. Treatment-emergent adverse events (TEAEs) until week 16 of deucravacitinib treatment in patients with psoriasis (n = 33).
Pruritus or elevation of creatinine phosphokinase occurred in two patients (6.1%), respectively. Noninfectious stomatitis was observed in one patient (3.0%). These AEs were mild in severity, and improved spontaneously or with appropriate medication. One AE (3.0%) leading to the discontinuation of deucravacitinib was the exacerbation of psoriatic arthritis. This case was improved by the administration of bimekizumab, a biologic inhibiting IL-17A and IL-17F.
Discussion
In this real-world study, the achievement rates of PASI 75 (78.3%), PASI 90 (52.2%), and PASI 100 (13.0%) at week 16 of deucravacitinib treatment were comparable to those observed in the Japanese subpopulation of clinical trials; 78.1%, 56.3%, and 25.0%, respectively (Citation6). Similarly, the achievement rate of DLQI 0/1 at week 16 in this study was 42.9%, which is in line with 45.2% in clinical trials. The reductions of PASI scores on four individual anatomical sites mostly paralleled with that of total PASI, indicating that deucravacitinib might universally improve the skin symptoms of psoriasis independently on the anatomical site. Further, the parallel reduction of total PASI and DLQI scores indicates that the improvement of skin rash by deucravacitinib treatment might simultaneously improve QOL of psoriasis patients.
Deucravacitinib treatment rapidly (within 2 weeks) and remarkably reduced PP-NRS in psoriasis patients with baseline pruritus. Clinical trials also showed that deucravacitinib reduced itch severity (Citation14). The percent reduction of PP-NRS was correlated with that of total PASI at weeks 4 and 8 of deucravacitinib treatment in this study. The results indicate that the improvement of pruritus by deucravacitinib might be pathomechanistically related to that of skin rash. Further, the percent reduction of DLQI, index of QOL, was correlated with that of PP-NRS at week 16, indicating that reduction of pruritus might improve QOL of the patients. Pruritus causes anxiety, depression, and sleep disturbance, and reduces labor or study productivity, leading to the deterioration of QOL.
How pruritus is generated and promoted in psoriasis lesions might be highly complex and has not been completely clarified (Citation15). Increased nerve density is reported in psoriasis skin lesions and considered to be one cause of chronic itch (Citation16,Citation17). According to itchscriptome comparing non-pruritic and pruritic lesions with psoriasis, pruritic lesions show the increased expression of genes for IL-23/17/22/31/36α/γ, TNF-α, transient receptor potential (TRP) vanilloid (V)1, TRPV3, TRP melastatin (M) 8, substance P, neurokin-1 receptor, mas-related G-protein-coupled receptor X2, etc., which may induce the itch sensation and hyperinnervation (Citation15). The expression of oncostatin M (OSM) is promoted in lesions with psoriasis, especially in CD4+ T cells, and might be involved in the psoriasis-associated pruritus (Citation18). Since TYK2 is involved in the signaling from IL-23 (Citation3) and OSM (Citation19), the itch-promoting effects of these cytokines might be directly attenuated by TYK2 inhibitor deucravacitinib.
The safety profile of deucravacitinib in the present real-world study was consistent with that in previous clinical trials, and no safety risks were newly observed (Citation4–6). Deucravacitinib exhibits high selectivity for TYK2 by allosterically inhibiting the pseudokinase domain of TYK2 without inhibiting JAK1-3 (Citation20). This selectivity may result in fewer AEs without altering blood cell counts or lipid parameters (Citation21–23), compared to traditional JAK inhibitors (Citation24–27). However, given that type I IFN pathway dependent on TYK2 signaling is important for host defense against infection (Citation28) and anti-tumor immunity (Citation29), careful assessment is required for the risk of infections and malignancies in longer-term treatment with deucravacitinib.
This study has several limitations. First, the sample size was small, and the study was conducted in a single center. Second, the duration of patients’ follow-up was short. Longer-term safety and effectiveness of deucravacitinib treatment should be examined in further studies. Third, the age distribution of the population in this study was rather old compared to Japanese population in clinical trials; the median [interquartile range] 60.0 [56.0–74.0] years in the former and mean 48.2 years in the latter (Citation6). The age distribution might influence the therapeutic effectiveness or safety profile. Lastly, we acknowledge the heterogeneity of our patient cohort in terms of clinical extent. This diversity posed a challenge, particularly in patients with reduced baseline PASI scores, where achieving PASI 75 and 90 responses may be more difficult compared to those with more extensive variants. Future studies should consider stratifying patients based on their baseline severity to better understand the efficacy of treatments across different patient subgroups.
Conclusions
In conclusion, deucravacitinib treatment for psoriasis in this real-world study was well tolerated and gave therapeutic effects and safety profile comparable with those in previous clinical trials. Deucravacitinib treatment rapidly improved pruritus in psoriasis patients.
Author contributions
Teppei Hagino conceptualized the study, and mainly organized the manuscript. Naoko Kanda supervised the study. Hidehisa Saeki and Eita Fujimoto revised the manuscript.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital (protocol code H-2023-095 and 7 December 2023 of approval).
Disclosure statement
T. H., H. S., E. F., and N. K. received lecture fees from Bristol Myers Squibb.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Ten Bergen LL, Petrovic A, Krogh Aarebrot A, et al. The TNF/IL-23/IL-17 axis-head-to-head trials comparing different biologics in psoriasis treatment. Scand J Immunol. 2020;92(4):1. doi: 10.1111/sji.12946.
- Hagino T, Saeki H, Fujimoto E, et al. Effects of biologic therapy on laboratory indicators of cardiometabolic diseases in patients with psoriasis. J Clin Med. 2023;12(5):1934. doi: 10.3390/jcm12051934.
- Schlapbach C, Conrad C. TYK-ing all the boxes in psoriasis. J Allergy Clin Immunol. 2022;149(6):1936–7. doi: 10.1016/j.jaci.2022.03.014.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39. doi: 10.1016/j.jaad.2022.07.002.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40–51. doi: 10.1016/j.jaad.2022.08.061.
- Imafuku S, Tada Y, Hippeli L, et al. Efficacy and safety of the selective TYK2 inhibitor, deucravacitinib, in Japanese patients with moderate to severe plaque psoriasis: subgroup analysis of a randomized, double-blind, placebo-controlled, global phase 3 trial. J Dermatol. 2023;50(5):588–595. doi: 10.1111/1346-8138.16740.
- Najar Nobari N, Shahidi Dadras M, Nasiri S, et al. Neutrophil/platelet to lymphocyte ratio in monitoring of response to TNF-α inhibitors in psoriatic patients. Dermatol Ther. 2020;33(4):e13457.
- Sen BB, Rifaioglu EN, Ekiz O, et al. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol. 2014;33(3):223–227. doi: 10.3109/15569527.2013.834498.
- Beygi S, Lajevardi V, Abedini R. C-reactive protein in psoriasis: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28(6):700–711. doi: 10.1111/jdv.12257.
- Kim DS, Shin D, Lee MS, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016;43(3):305–310. doi: 10.1111/1346-8138.13061.
- Hagino T, Saeki H, Kanda N. Biomarkers and predictive factors for treatment response to tumor necrosis factor-α inhibitors in patients with psoriasis. J Clin Med. 2023;12(3):974. doi: 10.3390/jcm12030974.
- Hagino T, Saeki H, Fujimoto E, et al. The eosinophil-to-lymphocyte ratio acts as an indicator for improvement of clinical signs and itch by upadacitinib treatment in atopic dermatitis. J Clin Med. 2023;12(6):2201. doi: 10.3390/jcm12062201.
- Gottlieb AB, Gordon K, Hsu S, et al. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2018;32(8):1305–1313. doi: 10.1111/jdv.14913.
- Armstrong A, Strober B, Gordon KB, et al., editors. Abstract No.:2127—deucravacitinib improves psoriasis symptoms and signs diary domain scores in patients with moderate to severe plaque psoriasis: results from the phase 3 POETYK PSO-1 and POETYK PSO-2 studies. EADV 30th Congress; 2021 Sep 29–Oct 2; virtual; 2021.
- Nattkemper LA, Tey HL, Valdes-Rodriguez R, et al. The genetics of chronic itch: gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J Invest Dermatol. 2018;138(6):1311–1317. doi: 10.1016/j.jid.2017.12.029.
- Taneda K, Tominaga M, Negi O, et al. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br J Dermatol. 2011;165(2):277–284. doi: 10.1111/j.1365-2133.2011.10347.x.
- Ayasse MT, Buddenkotte J, Alam M, et al. Role of neuroimmune circuits and pruritus in psoriasis. Exp Dermatol. 2020;29(4):414–426. doi: 10.1111/exd.14071.
- Tseng PY, Hoon MA. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci Transl Med. 2021;13(619):eabe3037. doi: 10.1126/scitranslmed.abe3037.
- Burfoot MS, Rogers NC, Watling D, et al. Janus kinase-dependent activation of insulin receptor substrate 1 in response to interleukin-4, oncostatin M, and the interferons. J Biol Chem. 1997;272(39):24183–24190. doi: 10.1074/jbc.272.39.24183.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62(20):8973–8995. doi: 10.1021/acs.jmedchem.9b00444.
- He X, Chen X, Zhang H, et al. Selective Tyk2 inhibitors as potential therapeutic agents: a patent review (2015–2018). Expert Opin Ther Pat. 2019;29(2):137–149. doi: 10.1080/13543776.2019.1567713.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs. 2020;80(4):341–352. doi: 10.1007/s40265-020-01261-8.
- Catlett IM, Hu Y, Gao L, et al. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J Allergy Clin Immunol. 2022;149(6):2010–2020.e8. doi: 10.1016/j.jaci.2021.11.001.
- Hagino T, Saeki H, Fujimoto E, et al. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023;50(7):869–879. doi: 10.1111/1346-8138.16763.
- Hagino T, Saeki H, Fujimoto E, et al. Background factors predicting the occurrence of herpes zoster in atopic dermatitis patients treated with upadacitinib. J Dermatol. 2023;50(10):1301–1312. doi: 10.1111/1346-8138.16879.
- Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167. doi: 10.1111/1346-8138.16549.
- Hagino THR, Yoshida M, Fujimoto E, et al. Effectiveness and safety of upadacitinib in combination with topical corticosteroids in adolescent patients with moderate-to-severe atopic dermatitis. Clin Cosmet Investig Dermatol. 2023;16:3201–3212. doi: 10.2147/CCID.S439053.
- Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570.
- Chen Z, Yin M, Jia H, et al. ISG20 stimulates anti-tumor immunity via a double-stranded RNA-induced interferon response in ovarian cancer. Front Immunol. 2023;14:1176103. doi: 10.3389/fimmu.2023.1176103.