Abstract
Background
The advent of biologics and janus kinase inhibitors has revolutionized treatment of atopic dermatitis (AD).
Objective
To investigate preferences of patients with AD for attributes of currently approved systemic treatments and assess influencing factors.
Methods
An online discrete choice experiment was conducted in patients with AD throughout Germany to analyze preferences for outcome (probability of (almost) clear skin at week 16, probability of significant itch improvement, time to onset of itch relief and type of side effects) and process attributes (application method and frequency of laboratory tests).
Results
Participants (n = 182, 75.3% female) considered side effects (Relative Importance Score (RIS): 31.2), (almost) clear skin (RIS: 24.2) and probability of itch improvement (RIS: 16.0) most important. Application method (RIS: 14.4), time to onset of itch relief (RIS: 7.4) and frequency of laboratory tests (RIS: 6.8) were less relevant. Preferences were significantly influenced by sex, age, psychiatric comorbidity, current therapy and health-related quality of life according to multivariate regression analysis.
Conclusions
Participants attached great importance to safety and symptom control. However, preferences were also dependent on individual characteristics, underscoring the importance of personal counseling. Conjoined with medical considerations, patients’ preferences have fundamental impact on shared decisions for treatment of AD.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease affecting approximately 15–20% of children and up to 10% of adults (Citation1). The clinical course is variable and characterized by recurrent relapses, with the majority of patients suffering from intense itch and pain in addition to the eczematous lesions (Citation2,Citation3). The disease may have profound impact on physical, emotional, and social well-being and entail a significant economic burden (Citation4,Citation5). Moreover, AD is associated with other atopic and non-atopic comorbidities (Citation6). Most cases of AD can be managed with topical treatments and/or phototherapy. However, if these therapies are not feasible or fail, systemic therapy is recommended, particularly in moderate-to-severe disease (Citation3,Citation7). Systemic corticosteroids can be considered as treatment for flares, but their administration is not encouraged for long-term use (Citation3). The oral calcineurin inhibitor cyclosporine A (CyA) was the only other systemic therapy approved for treatment of severe AD in Germany and several other countries for decades. Although CyA leads to a rapid and significant improvement, treatment is associated with serious side effects, including an increased risk of infections, nephrotoxicity, arterial hypertension, and malignancies in the long term (Citation3,Citation8). Therefore, this medication requires close monitoring and cannot be recommended as long-term strategy for AD (Citation7,Citation9).
With better understanding of molecular pathways, biologics targeting interleukin (IL)-4 and IL-13 and small molecules targeting the Janus kinase (JAK) Signal Transducer and Activator of Transcription (STAT) pathway have brought a new era in AD treatment. The IL-4 receptor antibody dupilumab was approved for treatment of moderate-to-severe AD in the United States (US) and in the European Union (EU) in 2017, the IL-13 antibody tralokinumab in 2021 and the IL-13 antibody lebrikizumab very recently in 2023 (Citation10–12). All biologics are administered subcutaneously at 14-day intervals during the first 16 weeks. Lebrikizumab is switched to 4-weekly injections after the induction phase (Citation13). A 4-weekly dosing regimen may also be considered for patients who achieve clear or almost clear skin after 16 weeks of treatment with tralokinumab (Citation14). Efficacy and safety of these biologics have been demonstrated in large clinical trials, real-world studies and registries (Citation14–17). Typical side effects of IL-4 and IL-13 antagonists are conjunctivitis and injection reactions. Apart from this, the safety profile is excellent, and routine laboratory monitoring is not required (Citation18,Citation19).
Modern oral therapies approved for moderate-to-severe AD are the JAK inhibitors abrocitinib, baricitinib and upadacitinib (Citation20). They provide a rapid onset of action and a robust and durable efficacy (Citation21). According to a systematic review with meta-analysis, abrocitinib 200 mg daily and upadacitinib 30 mg daily had the highest efficacy in reducing AD lesions during the first 16 weeks, followed by dupilumab and upadacitinib 15 mg, whereas tralokinumab and baricitinib were less effective (Citation22). Moreover, head-to-head studies showed more rapid skin clearance and itch relief with abrocitinib and upadacitinib compared to dupilumab (Citation23,Citation24). However, treatment with JAK inhibitors implies some safety concerns, which led to warnings of major cardiovascular adverse events, venous thromboembolism, severe infections and malignancies by drug authorities based on studies investigating tofacitinib in patients with rheumatological diseases (Citation25). According to a recent review on the safety of JAK inhibitors in dermatology, most common adverse events were infections, nausea, headache and acne (Citation26). Further side effects include herpes zoster, alterations in blood counts and elevation of liver enzymes and lipids, making laboratory monitoring mandatory (Citation7,Citation9). In addition to the currently approved medications, several new biologics and novel small molecules are under investigation and will hopefully expand the therapeutic landscape of AD in the future (Citation27,Citation28).
Guidelines for the treatment of AD serve as an important aid to orientation, but do not specify a clear sequence of therapies (Citation7,Citation9). Considering the rapidly expanding armamentarium of treatment options, it might be challenging to select the best treatment for an individual patient. The choice of therapy depends on disease characteristics (e.g., severity and course of the AD, affected body regions, current and previous treatments), patients’ characteristics (e.g., age, sex and comorbidities), properties of available medications (e.g., efficacy, safety, application mode, monitoring requirements and cost) and on physician-related aspects (e.g., experience and prescription preferences), but largely also on the individual preferences, wishes and concerns of the affected patient (Citation29).
Discrete choice experiments (DCEs) are a method to elicit preferences in a manner that imitates the clinical decision-making process. We previously used this approach to identify the preferences of patients with various dermatological diseases, including other chronic inflammatory dermatoses (Citation30–32). The aim of this study was to evaluate preferences of patients with AD for attributes of established and novel systemic therapies, to elucidate factors that influence these preferences and to aid shared decision-making between physicians and patients by this means.
Materials and methods
Study design
Patient preferences were assessed in a cross-sectional non-interventional online survey. Participants were recruited from April 2021 to March 2022 via announcements of German patient organizations for AD (Deutscher Neurodermitis Bund e.V., Bundesverband Neurodermitis e.V.) and via flyers distributed in dermatological departments of German hospitals. Inclusion criteria comprised physician-confirmed diagnosis of AD, age ≥18 years and informed consent to study participation. The study was performed according to the Declaration of Helsinki (Citation33) and was approved by the Ethics Committee of the Medical Faculty Mannheim (ethics approval 2021-507, 12 January 2021; amendment 09 November 2021).
Data collection
Data was collected via an online survey (Sawtooth software®, Provo, Utah, USA) which contained questions on demographics, medical history, comorbidity, and treatment of AD. Furthermore, participants were asked to rate the symptoms and signs of their AD. Disease severity and patient-reported outcomes (PROs) were assessed by the Patient-Oriented SCORing of Atopic Dermatitis tool (PO-SCORAD; range 0 (clear) to 103 (maximum severity)) (Citation34), the self-rated Body Surface Area (BSA; range 0–100%), the Patient-Oriented Eczema Measure (POEM; range 0 (clear) to 28 (very severe)) (Citation35), the peak pruritus intensity in the previous 24 h (h) (numeric rating scale (NRS) 0–10) (Citation36,Citation37), the average pruritus intensity in the previous 48 h (NRS 0–10) (Citation36,Citation37) as well as the average sleep disturbance in the previous 48 h (NRS 0–10) (Citation38). Further PROs included the Dermatology Life Quality Index (DLQI; range 0 (no effect on patient’s life) to 30 (extremely large effect)) (Citation39) and the Hospital Anxiety and Depression Scale (HADS; values for each subscale: 0–7: no, 8–10: borderline, and 11–21: significant anxiety or depression) (Citation40).
Discrete choice experiments
Choice-based conjoint analysis was performed with Sawthooth software for Hierarchical Bayes Estimation (for details, see https://content.sawtoothsoftware.com/assets/276545e9-0445-474c-b01c-f5b24c3eba6d). Discrete choice scenarios were created by decomposing systemic treatments for AD (CyA, biologics and JAK inhibitors) into 4 key outcome attributes (probability of (almost) clear skin at week 16, probability of significant itch improvement at week 16, time to onset of itch relief and type of side effects) and 2 process attributes (application method and frequency of laboratory tests). Three attribute levels depicting these medications as realistically as possible were chosen for each attribute (). Attributes and their levels were identified by literature research and discussion with experts (e.g., Citation41–43). Subsequently, pretest interviews were conducted with 10 patients who gave feedback on how they interpreted the text, with particular focus on comprehensibility of the attributes and levels and the DCE presented in the survey. DCE were designed with conjoint analysis software (Lighthouse Studio version 9.7.2, Sawtooth Software®, Provo, UT, USA). Attribute levels were randomly composed to choice experiments. The combination of the side effects ‘conjunctivitis, injection-site reaction, oral herpes’ with the application mode ‘tablets taken once to twice per day’ was prohibited by logical restriction. Participants were repeatedly asked to choose their preferred option in 13 randomly selected DCE with two pairwise presented scenarios (for an example see online supplementary Table S1). They were informed that some scenarios might be hypothetical. In order to ensure that the participants understood the DCE, two fixed experiments with one superior option were part of each survey.
Table 1. Attributes and attribute levels.
Statistical analysis
Statistical analysis was performed with commercially available software (Stata® version 17.0, StataCorp LLC, College Station, TX, USA). Figures were created with GraphPad Prism® version 9.0.0 (GraphPad Software, San Diego, CA, USA). Descriptive analysis was carried out for patient and disease characteristics and treatment regimens. Absolute and relative frequency tabulations described categorical variables. Mean, standard deviation, and range were reported for continuous variables. Correlation analysis for objective and subjective parameters of disease severity was performed using Spearman’s correlation. Logit regression analysis of the DCE data was conducted with Sawtooth software. A Part-Worth Utility (PWU) was derived for each attribute level, with positive values indicating utility and negative values indicating disutility. Relative Importance Scores (RIS) describing the level of preference were calculated from PWU of each participant and each attribute and then averaged across the cohort and in subgroups. Subgroup analysis with regard to sex, age, psychiatric comorbidity, current systemic treatment, experience with systemic therapy, DLQI and PO-SCORAD was performed with analysis of variance (ANOVA) followed by Bonferroni and Tukey’s Honestly Significant Difference post hoc tests. Multivariate regression analyses (Ordinary Least Squares method) were used to estimate the influence of sex, age, psychiatric comorbidity, current systemic treatment, DLQI and SCORAD on RIS and on the PWU of attributes with nominal scale (side effects and application method). Patients with missing values were excluded from all analyses. Significance was assumed at p < 0.05.
Results
Cohort characteristics
Overall, 194 AD patients completed the study. 12 participants were excluded due to wrong answers in the fixed control scenarios; thus, analysis was based on 182 individuals (). The majority of participants was female (75.3%), and the mean age of the cohort was 41.6 years. The most common comorbidities were atopic diseases other than AD (65.4%) and psychiatric disorders (18.7%). Among the 34 patients with psychiatric disorders, 27 suffered from depression, 15 from anxiety disorder and 1 from another psychiatric disorder that was not further specified. Almost all patients currently used topical agents, including moisturizing skin care products, and 11.0% received phototherapy. Approximately one in four patients was treated with systemic medication, mainly with biologics (18.1%; dupilumab: 17.6%; tralokinumab: 0.5%). Other systemic therapies were less common (oral glucocorticosteroids: 4.4%; JAK inhibitors: 2.7%; CyA: 0.5%).
Table 2. Characteristics of the study cohort.
The mean PO-SCORAD was 47.8, reflecting moderate disease severity (), even if uniform severity strata are not established (Citation44). The mean self-reported BSA was 37.5%. Patients assessed their eczema on average as moderate (15.6) according to the POEM (Citation35). However, 44.5% reached a POEM between 17 and 28, which indicates severe or very severe eczema (Citation35).
Table 3. Patient-reported outcomes.
The peak pruritus intensity in the previous 24 h was 4.7 (NRS 0–10) and the average pruritus intensity in the previous 48 h 4.4 (NRS 0–10). Disease symptoms led to sleep disturbance in the previous 48 h (4.0; NRS 0–10). The mean DLQI was 11.4, which translates to a very large effect on health-related quality of life (Citation45). The HADS showed at least suspected depression (defined as scores ≥8 (Citation40)) and at least suspected anxiety disorder (defined as scores ≥8 (Citation40)) in 88.0% and 73.7%, respectively. The assessments used to holistically characterize AD severity correlated well with each other (supplementary Table S2). A comparison of the PROs of participants with and without current systemic treatment is shown in supplementary Table S3. Patients currently receiving systemic medication had significantly lower peak and average pruritus, less sleep disturbance and a lower mean POEM than those without current systemic therapy.
Patient preferences
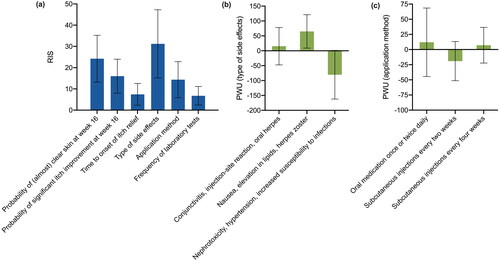
Averaged across the whole cohort, patients were most interested in the type of side effects (RIS: 31.2), followed by the probability of (almost) clear skin at week 16 (RIS: 24.2) and the probability of significant itch improvement at week 16 (RIS: 16.0; ). The application method (RIS: 14.4), time to onset of itch relief (RIS: 7.4), and frequency of laboratory tests (RIS: 6.8) were considered less relevant. Regarding side effects, participants were more interested in avoiding ‘nephrotoxicity, hypertension, and increased susceptibility to infections’ (PWU: −80.4) than avoiding ‘conjunctivitis, injection-site reaction, and oral herpes’ (PWU: 15.3) or ‘nausea, elevation in lipids, and herpes zoster’ (PWU: 65.1; ). As to the application method, patients favored an oral therapy (PWU: 12.0) more than injections every 4 weeks (PWU: 7.2) or every other week (PWU: −19.2; ). However, preferences were heterogeneous across the cohort, as illustrated by large standard deviations.
Figure 1. Preferences for systemic treatment of AD. Relative importance scores (RIS) averaged across the study cohort (a). PWU for type of side effects (b) and application method (c). Bars: means with standard deviations. AD: atopic dermatitis; PWU: Part-Worth Utility; RIS: Relative Importance Scores.
Influence of patient and disease characteristics on preferences
Descriptive analyses
Descriptive subgroup analysis showed no significant differences between the preferences of men and women (). Patients ≤40 years of age put more emphasis on clear skin (RIS: 26.7 vs. 22.0; p = 0.004) and itch improvement (RIS: 17.6 vs. 14.6; p = 0.012; ) than older participants. By contrast, the type of side effects was considered less relevant in the younger age group (RIS: 27.4 vs. 34.6; p = 0.002). Older patients were more concerned about nephrotoxicity, hypertension, and increased susceptibility to infections than younger ones (PWU: −97.2 vs. −61.7; p = 0.003) and more willing to accept nausea, elevation in lipids, and herpes zoster (PWU: 73.4 vs. 55.8; p = 0.035).
Figure 2. Treatment preferences according to sex, age and psychiatric comorbidity. (a) No significant differences were found with respect to sex. (b) Patients ≤ 40 years of age put more emphasis on the probability of clear skin (p = 0.004) and of itch improvement (p = 0.012) but regarded the type of side effect less relevant (p = 0.002). Older patients had greater concern about “nephrotoxicity, hypertension and increased susceptibility to infections” (p = 0.003) and were more willing to accept “nausea, elevation in lipids and herpes zoster” (p = 0.035). (c) Presence of a psychiatric comorbidity was associated with higher preferences for the probability of clear skin (p = 0.029) and less interest in the type of side effects (p = 0.020). Patients with psychiatric comorbidity were less afraid of “nephrotoxicity, hypertension and increased susceptibility to infections” (p = 0.008) and disliked injections every 4 weeks more (p = 0.033) than other participants. Bars: means with standard deviations. PWU: Part-Worth Utility; RIS: Relative Importance Scores. *p < 0.05, **p < 0.01.
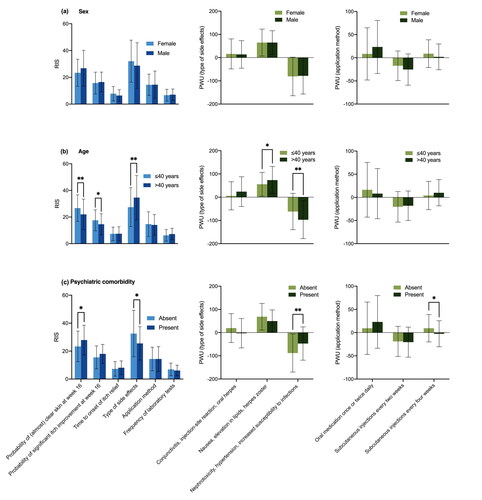
Presence of a psychiatric comorbidity was associated with greater interest in clear skin (RIS: 27.9 vs. 23.4; p = 0.029) and less focus on the type of side effects (RIS: 25.5 vs. 32.5; p = 0.020; ). Patients with psychiatric comorbidity were less afraid of nephrotoxicity, hypertension, and increased susceptibility to infections than patients without this comorbidity (PWU: −47.1 vs. −88.1; p = 0.008). In addition, they tended to favor an oral therapy more than others, and were significantly more disinclined to injections every 4 weeks (PWU: −2.5 vs. 9.4; p = 0.033).
Patients currently receiving non-biologic drugs placed significantly more value on the time to onset of itch relief compared to those treated with biologics (RIS: 12.4 vs. 6.9; p = 0.004) and those without systemic treatment (RIS: 12.4 vs. 7.1; p = 0.002; ). The subgroup with biologic therapy was more willing to accept ‘conjunctivitis, injection-site reaction, and oral herpes’ than those with non-biologic treatment or without systemic therapy (PWU: 45.8 vs. −27.5; p = 0.001; 45.8 vs. 11.7; p = 0.013). Avoiding nephrotoxicity, hypertension, and increased susceptibility to infections was particularly important for participants without systemic therapy and participants with biologics (PWU: −81.8 and −96.1 vs. −21.1 in patients on non-biologic drugs; p = 0.039 and p = 0.019). Moreover, patients treated with biologics were less interested in an oral therapy than those without systemic therapy (PWU: −15.0 vs. 17.4; p = 0.009) and favored an injection more (every 2 weeks: PWU: −4.8 vs. −21.6; p = 0.020; every 4 weeks: PWU: 19.7 vs. 4.2; p = 0.019). Participants receiving non-biologic agents disliked injections every other week more than those with biologics (PWU: −31.2 vs. −4.8; p = 0.042). Subgroup analysis performed to compare the preferences of patients who were experienced with systemic therapy (current or previous use; n = 86) or systemic treatment-naïve (n = 96) did not reveal significant differences (data not shown).
Figure 3. Treatment preferences dependent on current systemic therapy, DLQI and PO-SCORAD. (a) Patients receiving non-biologic drugs placed significantly more value on the time to onset of itch relief than others (p = 0.004 compared to patients on biologics; p = 0.002 compared to those without systemic treatment). Participants treated with biologics were more willing to accept “conjunctivitis, injection-site reaction and oral herpes” (p = 0.001 compared to non-biologic treatment; p = 0.013 compared to participants without systemic treatment). Those receiving non-biologic drugs were less interested in avoidance of “nephrotoxicity, hypertension and increased susceptibility to infections” (p = 0.039 compared to no systemic therapy; p = 0.019 compared to biologics). Compared to those without a systemic therapy, patients treated with biologics were less interested in an oral therapy (p = 0.009) and favored a subcutaneous treatment regimen more (every 2 weeks: p = 0.020; every 4 weeks: p = 0.019). Participants treated with non-biologic agents disliked a subcutaneous medication every other week more than those with biologics (p = 0.042). (b) Health-related quality of life had no significant impact on RIS. However, participants with a DLQI ≤10 attached greater importance to avoidance of “nephrotoxicity, hypertension and increased susceptibility to infections” (p = 0.015) and were more willing to accept “conjunctivitis, injection-site reaction and oral herpes” (p = 0.007). (c) PO-SCORAD >40 was associated with higher preferences for skin clearance (p = 0.015) and itch relief (p = 0.033) and less concern about the type of side effects (p = 0.026). Participants with a PO-SCORAD ≤40 worried more about “nephrotoxicity, hypertension and increased susceptibility to infections” (p = 0.004) and less about “conjunctivitis, injection-site reaction and oral herpes” (p = 0.015). Bars: means with standard deviations. DLQI: Dermatology Life Quality Index; PO-SCORAD: Patient-Oriented SCORing of Atopic Dermatitis; PWU: Part-Worth Utility; RIS: Relative Importance Scores. *p < 0.05, **p < 0.01.
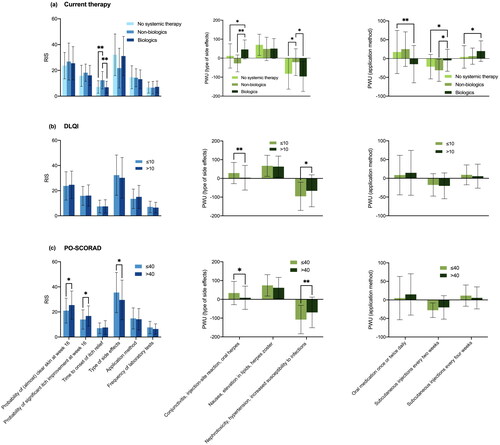
Health-related quality of life (DLQI) did not have a significant impact on RIS (). However, patients with lower DLQI (≤10) attached greater importance to avoiding ‘nephrotoxicity, hypertension, and increased susceptibility to infections’ and were more willing to accept ‘conjunctivitis, injection-site reaction, and oral herpes’ compared to participants with higher scores (PWU: −95.8 vs. −66.3; p = 0.015 and 28.6 vs. 3.4; p = 0.007, respectively). High disease activity (PO-SCORAD >40) was associated with higher preferences for skin clearance (RIS: 25.5 vs. 21.0; p = 0.015) and itch relief (RIS: 16.8 vs. 14.0; p = 0.033) and lower emphasis on the type of side effects (RIS: 29.6 vs. 35.5; p = 0.026; ). Those with lower disease activity (PO-SCORAD ≤ 40) were more concerned about ‘nephrotoxicity, hypertension and increased susceptibility to infections’ (PWU: −107.8 vs. −69.7; p = 0.004), and less worried about ‘conjunctivitis, injection-site reaction and oral herpes’ (PWU: 33.5 vs. 8.3; p = 0.015).
Multivariate regression analysis
Multivariate regression analysis was performed to estimate the impact of sex, age, psychiatric comorbidity, current systemic treatment, DLQI, and PO-SCORAD on RIS () and PWU of the type of adverse events and the treatment modality (). The preference for skin clearance was higher in males (ß: 4.594; p = 0.032) and patients ≤40 years of age (ß: 5.505; p = 0.001) compared to females and older participants, respectively. Moreover, younger participants placed more value on significant itch improvement at week 16 (ß: 3.168; p = 0.012) than older ones. The type of adverse events was more relevant to patients >40 years of age (ß: 8.048; p = 0.001) and less important to participants with psychiatric comorbidity (ß: −5.606; p = 0.031). Preferences for the time to onset of itch relief, the application method, and the frequency of laboratory tests were not significantly influenced by the prespecified independent variables.
Table 4. Multivariate linear regression models assessing the impact of patient and disease characteristics on RIS of treatment attributes.
Table 5. Multivariate linear regression models assessing the impact of patient and disease characteristics on PWU for levels of the attributes “type of side effects” and “application method”.
Regarding safety, higher DLQI scores predicted greater aversion to conjunctivitis, injection-site reaction, and oral herpes (ß: −2.025; p = 0.011). Patients treated systemically and younger patients were more interested in avoiding nausea, elevation in lipids, and herpes zoster than the respective reference groups (ß: −27.058; p = 0.007 and ß: −19.775; p = 0.025, respectively). Older patients particularly feared nephrotoxicity, hypertension, and increased susceptibility to infections (ß: −33.176; p = 0.007), while these adverse events were less relevant for patients with psychiatric comorbidity (ß: 32.670; p = 0.014) and higher DLQI scores (ß: 2.721; p = 0.005).
Concerning the application mode, males (ß: 21.341; p = 0.049) and patients without current systemic treatment (ß: 23.222; p = 0.023) favored tablets more than females and participants with systemic therapy. Higher preferences for injections every 4 weeks were observed in females (ß: 10.734; p = 0.046), patients without psychiatric comorbidity (ß: 11.913; p = 0.039) and patients receiving systemic therapy (ß: 12.539; p = 0.012), compared to the respective reference group.
Discussion
In the light of the rapidly evolving landscape of systemic treatments for moderate-to-severe AD, we assessed preferences for the most important attributes of all medications currently approved for this indication in Germany, including CyA, biologics, and JAK inhibitors. Averaged across the cohort, participants considered the safety of their treatment most important, followed by efficacy. To describe safety, we focused on common side effects of CyA, biologics and JAK inhibitors. These adverse events were presented in ‘packages’ in the DCE.
Boeri et al. (Citation46) conducted a DCE to identify preferences of adults with moderate-to-severe AD for systemic therapies in the US (n = 320, mean age: 35 years, 74% female). Safety-related attributes of their survey reflected rare serious adverse events characteristic of systemic immunosuppressants and JAK inhibitors prescribed for other systemic inflammatory diseases such as tofacitinib (Citation46). Due to a lack of reliable data on the incidence of these events in patients with AD and systemic therapy, they used data from patients with other inflammatory diseases to create attribute levels (Citation46). Their participants considered the annual risk of malignancy as the most important attribute, followed by mode of administration, and probability of clear skin at 16 weeks. Time to onset of itch relief and annual risks of serious infection or venous thromboembolism were regarded as less relevant. Even if Boeri and colleagues assessed rare life-threatening adverse events and we focused on more common, less serious side effects, both studies show high preferences for safety.
Okubo et al. (Citation47) examined the preferences of patients with moderate-to-severe AD (n = 323, mean age: 42.3 years, 51.7% female) and dermatologists (n = 121) for novel injectable treatments in a DCE in Japan. Their experiments contained the attributes ‘add-on or replacement treatment,’ ‘percentage improvement in severity of rashes,’ ‘reduction in itching,’ ‘time until response,’ ‘place of administration,’ ‘injection site reaction,’ ‘risk of mild to moderate side effects,’ ‘risk of severe side effects,’ ‘frequency of administration’ and ‘treatment cost per dose and per year.’ The side effects were not further specified, and the levels had a broad range (risk of mild to moderate side effects: 0–40%; risk of severe side effects: 0–5%). Participants set greatest importance on the risk of mild side effects, time until response and efficacy in reducing itch (Citation47), emphasizing again the high priority of safety and efficacy.
Kwatra et al. (Citation48) conducted a DCE in the US to measure patients’ willingness to trade off the risks and benefits of systemic treatments for moderate-to-severe AD. Participants (n = 200, mean age: 44 years, 59.5% female) rated improvement of itch, speed of itch reduction, and skin clearance as most important, while the annual risk of serious infections (0–5%) and acne (5–25%) as well as the need for concomitant prescription of topical corticosteroids were regarded as less relevant (Citation48).
Taken together, our study as well the aforementioned DCEs clearly and consistently illustrate that patients with AD attach great importance to attributes related to side effects and efficacy. However, it is difficult to compare the results directly, since the studies differ in the choice of attributes and levels, especially with regard to side effects. From our point of view, it is not possible to depict the side effects of the various classes of systemic therapies for AD absolutely realistically and with the complexity they deserve in DCE. While the biologics approved for AD show a rather uniform side effect profile in clinical studies, with nasopharyngitis, upper respiratory tract infection, conjunctivitis, injection site reactions, headache, and oral herpes as common adverse events (Citation18,Citation19,Citation49), the side effect profile of JAK inhibitors is more complex. According to a review on the safety of oral JAK inhibitors in dermatology, the most common adverse events with a frequency ≥5% in clinical trials included upper respiratory infections, nasopharyngitis, nausea, headache, and acne (Citation26). However, individual JAK inhibitors differ in their side effect profile (Citation50). As a result of studies investigating the pan-JAK inhibitor tofacitinib in rheumatological indications and subsequent warnings by heath authorities, the whole class of JAK inhibitors was afflicted with safety concerns such as the risk of serious infections, malignancies, venous thromboembolism, major adverse cardiac events, and mortality (Citation25). Patients with AD have to be informed accurately about the safety profile of all systemic treatment approaches suitable in their situation with particular emphasis on common and on serious adverse events, however without evoking exaggerated fear. Their baseline risk factors need to be carefully assessed, critically discussed, and weighed against the benefits of the treatment during shared decision-making in order to identify the therapy with the best risk-benefit profile, treatment comfort and fit to individual needs (Citation50).
Apart from safety, it is not surprising that efficacy was identified as an important attribute in all mentioned DCE. Head-to-head studies comparing dupilumab to upadacitib (Citation23) or to abrocitinib (Citation24) revealed a significantly faster onset of action of the JAK inhibitors, but comparable efficacy in achieving skin clearance in the longer term. In line with this, network meta-analyses demonstrated that a larger proportion of patients reached 75% or 90% improvement of AD lesions with upadacitinib und abrocitinib than with biologics during the induction phase (Citation21,Citation51,Citation52). However, the efficacy of JAK inhibitors and biologics approaches during longer term maintenance treatment (Citation53).
Even though the attribute ‘application method’ reached only moderate importance in our study, participants clearly preferred a tablet or an injection every 4 weeks over an injection every 2 weeks. This observation is in line with the mentioned study by Boeri et al. (Citation46), in which respondents preferred a daily pill to an injection every 2 weeks. In adults, all biologics approved for AD are administered subcutaneously every other week in the induction phase (Citation7,Citation54). However, the dosing regimen of tralokinumab is flexible thereafter, allowing 4-weekly injections in patients who achieved clear or almost clear skin, and injection intervals of lebrikizumab are routinely extended to every 4 weeks after 16 weeks of therapy (Citation54). In a cross-sectional study assessing the preferred mode of therapy in patients with rheumatoid arthritis, the oral route was also identified as the most preferred application mode, followed by subcutaneous injection (Citation55). Reasons stated for this preference were ease of application, speed of administration, and portability of the medication (Citation55). According to these and our results and in line with clinical experience, individual patients attach great importance to obtaining an oral treatment. These patients will likely choose JAK inhibitors as first-line systemic therapy for their moderate-to-severe AD if feasible from the medical point of view.
In a recent DCE on patients’ preferences for attributes associated with treatments for mild-to-moderate AD including topical agents, participants preferred a cream twice daily over a pill or tablet once daily and a subcutaneous self-injection once every other week (Citation56), indicating that topical treatment is regarded as an attractive application method in mild-to-moderate AD. Subgroup analysis revealed that respondents with lower self-assessed disease burden were more likely to prefer topical over systemic treatments than participants with higher disease burden (Citation56). This is plausible as topical medications are sufficient to control the vast majority of cases with mild AD but sometimes not efficient enough in moderate-to-severe disease.
Several patient, disease and treatment characteristics influenced the preferences of our participants. Regression analyses suggested that males were more interested in skin clearance and oral therapy than females. Boeri et al. (Citation46) did not find significant differences between preferences with respect to gender using a random-parameters logit model. Males had no higher odds of opting-in to a new treatment compared to females, regarding the study by Okubo et al. (Citation47). Older participants were willing to trade efficacy for safety, consistent with results of our previous studies investigating preferences for psoriasis treatments (Citation30,Citation57). Older patients are more prone to comorbidity and polypharmacy (Citation58). Therefore, they may be less willing to accept additional risks due to treatment of their AD. Furthermore, higher age was associated with a higher disutility for the side effect level ‘nephrotoxicity, hypertension, and increased susceptibility to infections.’ This is plausible because older participants have a higher baseline risk of renal impairment, hypertension and serious infections and therefore set high value on avoiding additional organ damage, cardiovascular risk and risk of infection. Indeed, CyA is often not suitable in older people due to comorbidity, and JAK inhibitors should be used with caution at ≥65 years of age. In the tradeoff against other side effects, older participants were more prepared to accept nausea, elevation in lipids, and a certain risk of herpes zoster. Nausea and an increase in blood lipids are likely perceived as less severe than nephrotoxicity and infections. The fear of herpes zoster was possibly limited, because there is a vaccine against this disease (Shingrix®), which is recommended by the German Standing Committee on Vaccination (STIKO) for all people aged ≥60 years and for immunocompromised persons from an age of 50 (Citation59).
Interestingly, study participants with psychiatric comorbidity considered the efficacy in skin clearance as more and safety as less important compared to other participants. Furthermore, they feared nephrotoxicity, hypertension, and increased susceptibility to infections less and disapproved injection every 4 weeks more than others. There is strong evidence that AD has a significant detrimental impact on mental health (Citation6). Studies indicate that depressive symptoms may be directly related to AD severity and may decrease upon improvement of AD (Citation6). It is well conceivable that our participants with psychiatric comorbidity appreciated a highly efficient AD treatment even more, as they also took benefit from it in terms of the psychiatric burden. In order to receive the treatment with the best efficacy, they may be willing to trade safety aspects.
Current systemic therapy had a significant influence on preferences for the type of side effects and the application method. According to regression analyses, patients treated with systemic medications showed a higher disutility for safety features describing JAK inhibitors and for oral medications compared to others, but favored injections every 4 weeks. When interpreting these data, it has to be kept in mind that most participants with systemic therapy received biologics. Our results suggest that this subgroup was rather satisfied with their injectable therapies but preferred longer application intervals. This preference should be considered in the choice of therapy and during the treatment course. Regarding side effects, patients who have opted for biologics may have chosen these medications due to their favorable safety profile and concerns about side effects of JAK inhibitors. The assumption that they were relatively satisfied with the safety profile and application mode of biologics is also supported by the results of the descriptive analyses in which the subgroup on biologics was considered separately.
Patients with a DLQI ≤ 10 attached greater importance to avoiding side effects typical of CyA (nephrotoxicity, hypertension, and increased susceptibility to infections) and were more willing to accept conjunctivitis, injection-site reactions, and oral herpes than those with a higher DLQI. The side effects mentioned first are more dangerous and more frequently irreversible than the latter ones. It is well conceivable that patient with little or moderate health-related life quality impairment are unwilling to risk threatening and potentially irreversible adverse events.
Those with a PO-SCORAD > 40 were particularly interested in skin clearance and itch control and placed less emphasis on side effects than those with a lower score according to descriptive analyses, indicating that severely affected participants were willing to accept side effects to achieve symptom control.
Certainly, it is essential to reevaluate patients’ preferences and needs during the course of therapy and to adapt the treatment, if required. Both biologics with flexible dosing regimens and JAK inhibitors are interesting in this regard. The effectiveness and certain side effects of JAK inhibitors are dose-dependent (Citation21,Citation26), and physicians should consider using approved dosage flexibilities where appropriate. We assume that sequential therapy, intermittent treatment pauses and dose flexibility will be important questions in future research on patient-centered care of AD.
Several limitations should be noted when interpreting our results. Studies have shown that patients with AD value a variety of attributes when choosing their therapy (Citation60). Due to the method and the data available at the time the study was designed, we were only able to consider a selected number of attributes and levels. Thus, it is possible that not all relevant attributes or attribute levels were taken into account in the DCE. For example, time to relapse was not incorporated, and not all rare but serious side effects could be considered. Oral herpes is described as a side effect of biologics in the medicinal product information, but recent studies argue against an increased risk of herpes upon biologic therapy of AD (Citation61). Although we picked the clinically most important variables in the regression model, endogeneity cannot be completely ruled out, which might bias results. Our DCE was designed to assess patient preferences in the induction phase of the therapy. Preferences, efficacy outcomes and risks may be somewhat different in the maintenance phase. Furthermore, our cohort size was limited. In particular, the subgroup of participants receiving systemic therapy was small. In patients with current systemic therapy, treatment duration was not assessed. Data were self-reported and not verified by clinical assessment. Finally, patient preferences may differ when making actual treatment decisions, which is a general limitation of DCE, and we cannot ensure that the attributes and levels were interpreted correctly by all participants.
Our study was designed and conducted independent of the pharmaceutical industry. Major advantages are consideration of all systemic medications currently approved for treatment of AD in Germany, including CyA, biologics, and JAK inhibitors, integration of various important attributes and realistic adaptation of attribute levels to approved medications. This allows a good transfer of our findings into clinical practice.
In conclusion, our participants set top priority on safety of their therapy, followed by efficacy in terms of skin clearing and improvement of itch. These results emphasize the importance of discussing the specific risk-benefit profile of the systemic therapies when it comes to personalized treatment selection for patients with AD. Furthermore, we identified several characteristics that influenced preferences, which additionally illustrates the obligation of an individualized treatment plan. Our results may aid shared decision-making between clinicians and patients when different systemic treatment options for moderate-to-severe AD are suitable.
Authors contributions
MLS, CK and WKP were involved in the conception and design of the study. DK, PW and CK performed analysis. MLS, DK, WKP, PW and CK interpretated the data. MLS, CK and WKP prepared the first draft of the paper. MLS, DK, WKP, PW and CK revised it critically for intellectual content and approved the final version to be published. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download PDF (526 KB)Acknowledgements
The authors would like to thank all participants. For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme „Open Access Publikationskosten“ as well as by Heidelberg University.
Disclosure statement
M.-L. Schaarschmidt has been an advisor to and/or received speakers’ honoraria from and/or received grants from and/or participated in clinical trials by the following companies: Abbvie, Allmirall, Biogen Inc., BMS GmbH, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen-Cilag GmbH, LEO Pharma, Merck Serono GmbH, MSD SHARP & DOHME GmbH, Novartis Pharma GmbH, Sanofi and UCB. W. K. Peitsch served as advisor for and/or obtained speakers’ honoraria from and/or received grants from and/or participated in clinical trials by the following companies: AbbVie, ALK-Abello, Almirall Hermal, Array Biopharma, Beiersdorf, Biotest, BMS, Boehringer Ingelheim, Celgene, Dermapharm, Dermasence, Eli Lilly, Galderma, GSK, Janssen-Cilag, L’Oreal, La Roche Posay, LEO Pharma, Medac, MSD, Novartis, Pfizer, Dr. Pfleger, Pierre Fabre, P&M Cosmetics, Roche, Sanofi, Sun Pharma and UCB Pharma. C. Kromer has been an advisor to and/or received speakers’ honoraria from and/or received grants from Janssen-Cilag, Novartis, and Boehringer Ingelheim. D. Kromer and P. Wellmann declare no conflicts of interest. The disclosed conflicts of interest have not influenced the content of this manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Additionally, the used model can be provided upon request.
Additional information
Funding
References
- Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990-2017. Br J Dermatol. 2021;184(2):1–12. doi: 10.1111/bjd.19580.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Pain is a common and burdensome symptom of atopic dermatitis in United States adults. J Allergy Clin Immunol Pract. 2019;7(8):2699–2706.e2697. doi: 10.1016/j.jaip.2019.05.055.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi: 10.1016/S0140-6736(20)31286-1.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104(3):174–177.
- Hebert AA, Stingl G, Ho LK, et al. Patient impact and economic burden of mild-to-moderate atopic dermatitis. Curr Med Res Opin. 2018;34(12):2177–2185. doi: 10.1080/03007995.2018.1498329.
- Thyssen JP, Halling AS, Schmid-Grendelmeier P, et al. Comorbidities of atopic dermatitis-what does the evidence say? J Allergy Clin Immunol. 2023;151(5):1155–1162. doi: 10.1016/j.jaci.2022.12.002.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. doi: 10.1111/jdv.18345.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133(2):429–438. doi: 10.1016/j.jaci.2013.07.049.
- Werfel T, Heratizadeh A, Aberer W, et al. S3-Leitlinie “Atopische Dermatitis” (AWMF-Registernr. 013-027). 2023. Available from: https://register.awmf.org/de/leitlinien/detail/013-027.
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi: 10.1111/bjd.19574.
- Silverberg JI, Guttman-Yassky E, Thaçi D, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388(12):1080–1091. doi: 10.1056/NEJMoa2206714.
- Blauvelt A, Thyssen JP, Guttman-Yassky E, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. 2023;188(6):740–748. doi: 10.1093/bjd/ljad022.
- Singh R, Taylor A, Shah MA, et al. Review of tralokinumab in the treatment of atopic dermatitis. Ann Pharmacother. 2023;57(3):333–340. doi: 10.1177/10600280221105686.
- Halling AS, Loft N, Silverberg JI, et al. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84(1):139–147. doi: 10.1016/j.jaad.2020.08.051.
- Sedeh FB, Henning MAS, Jemec GBE, et al. Comparative efficacy and safety of monoclonal antibodies and janus kinase inhibitors in moderate-to-severe atopic dermatitis: a systematic review and meta-analysis. Acta Derm Venereol. 2022;102:adv00764. doi: 10.2340/actadv.v102.2075.
- Stölzl D, Sander N, Heratizadeh A, et al. Real-world data on the effectiveness, safety and drug survival of dupilumab: an analysis from the TREATgermany registry. Br J Dermatol. 2022;187(6):1022–1024. doi: 10.1111/bjd.21794.
- Beck LA, Thaçi D, Deleuran M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an Open-Label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2020;21(4):567–577. doi: 10.1007/s40257-020-00527-x.
- Simpson EL, Merola JF, Silverberg JI, et al. Safety of tralokinumab in adult patients with moderate-to-severe atopic dermatitis: pooled analysis of five randomized, double-blind, placebo-controlled phase II and phase III trials. Br J Dermatol. 2022;187(6):888–899. doi: 10.1111/bjd.21867.
- Huang IH, Chung WH, Wu PC, et al. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: an updated review. Front Immunol. 2022;13:1068260. doi: 10.3389/fimmu.2022.1068260.
- Silverberg JI, Hong HC, Calimlim BM, et al. Comparative efficacy of targeted systemic therapies for moderate-to-severe atopic dermatitis without topical corticosteroids: an updated network meta-analysis. Dermatol Ther. 2023;13(10):2247–2264. doi: 10.1007/s13555-023-01000-3.
- Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523–532. doi: 10.1001/jamadermatol.2022.0455.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023.
- Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–282. doi: 10.1016/S0140-6736(22)01199-0.
- Kragstrup TW, Glintborg B, Svensson AL, et al. Waiting for JAK inhibitor safety data. RMD Open. 2022;8(1):e002236. doi: 10.1136/rmdopen-2022-002236.
- Samuel C, Cornman H, Kambala A, et al. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther. 2023;13(3):729–749. doi: 10.1007/s13555-023-00892-5.
- David E, Ungar B, Renert-Yuval Y, et al. The evolving landscape of biologic therapies for atopic dermatitis: present and future perspective. Clin Exp Allergy. 2023;53(2):156–172. doi: 10.1111/cea.14263.
- Tsuji G, Yamamura K, Kawamura K, et al. Novel therapeutic targets for the treatment of atopic dermatitis. Biomedicines. 2023;11(5):1303. doi: 10.3390/biomedicines11051303.
- Traidl S, Heratizadeh A. Modern systemic therapies for atopic dermatitis: which factors determine the choice of therapy? Dermatologie. 2022;73(7):529–537. doi: 10.1007/s00105-022-05003-7.
- Kromer C, Schaarschmidt ML, Schmieder A, et al. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS One. 2015;10(6):e0129120. doi: 10.1371/journal.pone.0129120.
- Schaarschmidt ML, Herr R, Gutknecht M, et al. Patients’ and physicians’ preferences for systemic psoriasis treatments: a nationwide comparative discrete choice experiment (PsoCompare). Acta Derm Venereol. 2018;98(2):200–205. doi: 10.2340/00015555-2834.
- Faverio K, Peitsch WK, Görig T, et al. Patient preferences in hidradenitis suppurativa (APProach-HS): a discrete choice experiment. J Dtsch Dermatol Ges. 2022;20(11):1441–1452. doi: 10.1111/ddg.14886.
- World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053.
- Stalder JF, Barbarot S, Wollenberg A, et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy. 2011;66(8):1114–1121. doi: 10.1111/j.1398-9995.2011.02577.x.
- Charman CR, Venn AJ, Ravenscroft JC, et al. Translating patient-oriented eczema measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol. 2013;169(6):1326–1332. doi: 10.1111/bjd.12590.
- Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502–507. doi: 10.2340/00015555-1246.
- Verweyen E, Ständer S, Kreitz K, et al. Validation of a comprehensive set of pruritus assessment instruments: the chronic pruritus tools questionnaire PRURITOOLS. Acta Derm Venereol. 2019;99(7):657–663. doi: 10.2340/00015555-3158.
- Narla S, Silverberg JI. Which clinical measurement tools for atopic dermatitis severity make the most sense in clinical practice? Dermatitis. 2023. Online ahead of print. doi: 10.1089/derm.2022.0087.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x.
- Newsom M, Bashyam AM, Balogh EA, et al. New and emerging systemic treatments for atopic dermatitis. Drugs. 2020;80(11):1041–1052. doi: 10.1007/s40265-020-01335-7.
- Nezamololama N, Fieldhouse K, Metzger K, et al. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib. Drugs Context. 2020;9:1–7. doi: 10.7573/dic.2020-8-5.
- Puar N, Chovatiya R, Paller AS. New treatments in atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):21–31. doi: 10.1016/j.anai.2020.08.016.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2018;121(4):464–468.e463. doi: 10.1016/j.anai.2018.07.004.
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664. doi: 10.1111/j.0022-202X.2005.23621.x.
- Boeri M, Sutphin J, Hauber B, et al. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatolog Treat. 2022;33(3):1449–1458. doi: 10.1080/09546634.2020.1832185.
- Okubo Y, Ho KA, Fifer S, et al. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatolog Treat. 2020;31(8):821–830. doi: 10.1080/09546634.2019.1623860.
- Kwatra SG, Lio P, Weidinger S, et al. Patient preferences for atopic dermatitis treatments: a discrete choice experiment. J Dermatolog Treat. 2023;34(1):2222201.
- Blauvelt A, Langley RG, Lacour JP, et al. Long-term 2-year safety and efficacy of tralokinumab in adults with moderate-to-severe atopic dermatitis: interim analysis of the ECZTEND open-label extension trial. J Am Acad Dermatol. 2022;87(4):815–824. doi: 10.1016/j.jaad.2022.07.019.
- Wood H, Chandler A, Nezamololama N, et al. Safety of janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61(6):746–754. doi: 10.1111/ijd.15853.
- Silverberg JI, Hong HC, Thyssen JP, et al. Comparative efficacy of targeted systemic therapies for moderate to severe atopic dermatitis without topical corticosteroids: systematic review and network meta-analysis. Dermatol Ther. 2022;12(5):1181–1196. doi: 10.1007/s13555-022-00721-1.
- Drucker AM, Lam M, Elsawi R, et al. Comparing binary efficacy outcomes for systemic immunomodulatory treatments for atopic dermatitis in a living systematic review and network meta-analysis. Br J Dermatol. 2023: ljad393. Online ahead of print. doi: 10.1093/bjd/ljad393.
- Silverberg JI, Armstrong A, Blauvelt A, et al. Assessment of efficacy and safety outcomes beyond week 16 in clinical trials of systemic agents used for the treatment of moderate to severe atopic dermatitis in combination with topical corticosteroids. Am J Clin Dermatol. 2023;24(6):913–925. doi: 10.1007/s40257-023-00809-0.
- Duggan S. Tralokinumab: first approval. Drugs. 2021;81(14):1657–1663. doi: 10.1007/s40265-021-01583-1.
- Bukhari RI, Alamr R, Alsindi RA, et al. Preferred mode of therapy among patients in rheumatoid arthritis saudi database: a cross-sectional study. Cureus. 2023;15(6):e41014. doi: 10.7759/cureus.41014.
- Myers K, Silverberg JI, Parasuraman S, et al. Treatment preferences among patients with mild-to-moderate atopic dermatitis. J Dermatolog Treat. 2023;34(1):2215356.
- Schaarschmidt ML, Schmieder A, Umar N, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011;147(11):1285–1294. doi: 10.1001/archdermatol.2011.309.
- Daunt R, Curtin D, O’Mahony D. Polypharmacy stewardship: a novel approach to tackle a major public health crisis. Lancet Healthy Longev. 2023;4(5):e228–e235. doi: 10.1016/S2666-7568(23)00036-3.
- Werner RN, Ghoreschi K. Herpes zoster-prevention, diagnosis, and treatment. Hautarzt. 2022;73(6):442–451. doi: 10.1007/s00105-022-04992-9.
- Ervin C, Crawford R, Evans E, et al. Patient and caregiver preferences on treatment attributes for atopic dermatitis. J Dermatolog Treat. 2022;33(4):2225–2233. doi: 10.1080/09546634.2021.1940810.
- Blauvelt A, Wollenberg A, Eichenfield LF, et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2023;40(1):367–380. doi: 10.1007/s12325-022-02322-y.

