Abstract
Background
Janus kinase 1 inhibitor upadacitinib is therapeutically effective for atopic dermatitis (AD). However, predictive factors for high responders to upadacitinib have not been established in real-world clinical practice.
Objectives
To identify predictive factors for responders to upadacitinib 15 mg or 30 mg, defined as achievers of investigator’s global assessment (IGA) 0/1 with ≥ 2-point improvement from basal IGA.
Methods
A retrospective study was conducted from August 2021 to July 2023 on 159 AD patients treated with upadacitinib 15 mg and 52 patients with 30 mg. Patients in each group were categorized into responders (achievers of IGA 0/1 at week 12) and non-responders (non-achievers). We compared baseline values of clinical and laboratory parameters between responders and non-responders. Logistic regression analysis was used to detect variables predicting responders. Receiver-operating characteristic curves were used for evaluating prediction capabilities of the variables.
Results
In logistic regression analysis, responders to 15 mg upadacitinib were associated with lower total EASI and higher age whereas responders to 30 mg were associated with lower LDH and lower IgE.
Conclusions
Lower total EASI and higher age may predict responders to upadacitinib 15 mg while lower IgE and lower LDH may predict responders to 30 mg.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by type 2-skewed abnormal immune responses, itching, and impaired skin barrier (Citation1–3). Research has indicated the involvement of certain cytokines, including interleukin (IL)-4, IL-13, IL-22, thymic stromal lymphopoietin, or IL-31, in the pathogenesis of AD, which transduce intracellular signals through the Janus kinase (JAK)/signal transducer and activator of transcription pathway (Citation4–7). In the current treatment landscape of Japan, three oral JAK inhibitors, upadacitinib, baricitinib, and abrocitinib, are approved for AD. Among these, upadacitinib has shown significant therapeutic effectiveness for moderate-to-severe AD, both in clinical trials and real-world clinical practice (Citation8–20). However, the treatment responsiveness to upadacitinib is heterogenous in AD patients, and not all patients favorably respond to this medicine in real-world clinical practice. It is thus highly useful to identify the characteristics of responders to upadacitinib, such as background factors or baseline levels of clinical or laboratory indexes. Such characteristics will help clinicians apply upadacitinib only to the patients predicted as its responders, and avoid wasteful usage of this medicine with high-price.
Our previous study revealed that the predictive factor for high percent reduction of eczema area and severity index (EASI) at week 4 or 12 was high baseline total eosinophil count (TEC) or female sex, respectively (Citation8). However, our previous study showed percent reduction of EASI, a measure that inherently depends on baseline EASI scores. High baseline EASI scores can lead to a larger percent reduction of EASI, while low baseline EASI scores can result in a smaller percent reduction of EASI.
In addressing the observations from our previous study, we recognize the need for clarification on the objective and significance of the current study. Although the analytical methods differ, the aim remains to identify predictive factors for responders to upadacitinib. The apparent discrepancies may be attributed to differences in patient backgrounds, sample sizes, and analytical approaches. The current study boasts a substantially larger sample size, which may influence consistency with our previous findings.
In this study, to overcome this limitation and standardize treatment outcomes, we aimed to identify predictive factors for responders to upadacitinib, defined as achievers of investigator’s global assessment (IGA) 0/1 response (clear/almost clear skin) with ≥ 2-point improvement from baseline IGA in real-world clinical practice.
Methods
Study design and data collection
From August 2021 to July 2023, 211 Japanese patients (aged ≥ 12 years) with moderate-to-severe AD (eczema area and severity index [EASI] ≥ 16 or EASI of head and neck ≥ 2.4) were treated in our department with oral upadacitinib 15 mg/day or 30 mg/day. Moderate-to-strongest classes of topical corticosteroids were administered twice daily concomitantly in all the patients. The diagnosis of AD was made clinically based on the Japanese Atopic Dermatitis Guidelines 2021 (Citation21). Medical records were examined retrospectively. This study was conducted based on the Declaration of Helsinki (2004) and was approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital. Patients provided written informed consent.
Data collection
For both upadacitinib 15 mg and 30 mg treatment groups, baseline characteristics were recorded and evaluated. These characteristics included sex, age, disease duration of AD, body mass index (BMI), history of bronchial asthma (BA), allergic conjunctivitis, or allergic rhinitis, previous treatment with dupilumab, upadacitinib 15 mg, or baricitinib 4 mg, total (whole body) EASI scores, EASI scores of four anatomical sites (head and neck, trunk, upper limbs, and lower limbs), IGA, peak pruritus-numerical rating scale (PP-NRS), and values of immunoglobulin E (IgE), thymus and activation-regulated chemokine (TARC), lactate dehydrogenase (LDH), blood counts of eosinophils, neutrophils, lymphocytes, platelets, monocytes, eosinophil-to-lymphocyte ratio (ELR), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR).
Patients in each treatment group were categorized into achievers of IGA 0/1 at week 12 (responders) and non-achievers (non-responders). Baseline values of clinical indexes or laboratory parameters or other background factors were compared between responders versus non-responders in each treatment group.
Statistical analysis
Results are expressed as mean ± standard deviation for variables with a normal distribution, and as the median and interquartile range for variables with a nonparametric distribution. Fisher’s exact test was used to assess the significance of differences in the frequency distributions. Differences between the two groups were assessed using Student’s t-test for variables with a normal distribution, and Mann-Whitney U test for variables with a non-parametric distribution. Statistical significance was set at p < 0.05.
The association between responder status and each variable was evaluated using bivariate and multivariate logistic regression analyses. These analyses included only the variables with a p < 0.05 in univariate analyses, adjusted for age, sex and BMI. We excluded variables with a variance inflation factor > 10 to avoid multicollinearity. Receiver-operating characteristic (ROC) analysis was performed to evaluate the prediction capabilities of variables for responders. The area under the curve (AUC) was used to judge the prediction capabilities. We performed all statistical analyses using EZR (Saitama Medical Center, Jichi Medical School).
Results
Baseline characteristics of upadacitinib 15 mg and 30 mg treatment groups
In this study, we analyzed 211 Japanese patients with moderate-to-severe AD (). Of these, 159 patients were treated with upadacitinib 15 mg and 52 with 30 mg. Before treatment, BMI, disease duration, the rates of BA, prior usage of dupilumab, upadacitinib 15 mg, and baricitinib, and values of IgE, TARC, numbers of neutrophils, platelets, and PLR were higher while values of IGA, total EASI, EASI of trunk, upper, and lower limbs, PP-NRS were lower in 30 mg group compared to 15 mg group. These data of background characteristics indicate that 30 mg treatment group may be the population with higher type 2 immune responses with allergic background while 15 mg group may be the population with more severe clinical signs and pruritus. The lower severity in clinical indexes in 30 mg group compared to 15 mg group may be possibly due to the high rate of prior usage of systemic treatments such as dupilumab or JAK inhibitors.
Table 1. Baseline demographic and disease characteristics of patients with atopic dermatitis (n = 211).
Responders in each treatment group
At week 12 of upadacitinib treatment, achievers of IGA 0/1 (responders) were 35 out of 159 patients (22.0%) in 15 mg group and 15 out of 52 patients (28.8%) in 30 mg group, and there was no difference in the percentage of responders between the two groups (p = 0.349 by Fisher’s exact test).
Predictive factors for responders to upadacitinib 15 mg treatment
In 15 mg treatment group, responders showed lower baseline total EASI, EASI of head and neck, PP-NRS, IgE, numbers of neutrophils, monocytes, and MLR compared to non-responders (). Multivariate logistic regression analysis revealed that the responder to 15 mg upadacitinib was associated with higher age (odds ratio, 1.03; 95% CI, 1.0–1.06; p = 0.0483) and lower baseline total EASI (odds ratio, 0.931; 95% CI, 0.874–0.992; p = 0.00275) ().
Table 2. Comparison of the background factors between responders and non-responders to upadacitinib 15 mg or 30 mg treatment for atopic dermatitis.
Table 3. Multiple logistic regression analysis for the association of each variable with responders to treatment with upadacitinib 15 mg (n = 159) or 30 mg (n = 52) for atopic dermatitis.
Predictive factors for responders to upadacitinib 30 mg treatment
In 30 mg upadacitinib group, responders showed lower baseline levels of IgE, TARC, LDH, and number of eosinophils compared to non-responders (). Multivariate logistic regression analysis revealed that the responder to 30 mg upadacitinib was associated with lower baseline IgE (odds ratio, 1.0; 95% CI, 1.0–1.0; p = 0.0472) and lower baseline LDH (odds ratio, 0.983; 95% CI, 0.966–0.999; p = 0.0381) ().
We then analyzed the prediction capabilities of those variables for responders to upadacitinib 15 mg or 30 mg by ROC curves. ROC curves demonstrated that prediction of responders to upadacitinib 15 mg could be achieved by a cutoff value 24 of baseline total EASI, with sensitivity 71.4%, specificity 58.9%, and AUC 0.638 (), indicating a low prediction capability of baseline total EASI. Additionally, the ROC analysis demonstrated that prediction of responders to upadacitinib 15 mg could be achieved by a cutoff value 37 years of age, with sensitivity 54.3%, specificity 58.9%, and AUC 0.55 (), indicating a very poor prediction capability of age.
Figure 1. The receiver operating characteristic (ROC) curves for evaluating prediction capabilities of individual parameters for responders to upadacitinib 15 mg or 30 mg treatment. The figures present prediction capabilities of baseline total EASI (a) or age (b) for IGA 0/1 response to 15 mg upadacitinib at week 12, and those of baseline IgE (c) and LDH (d) for the same response to 30 mg upadacitinib.
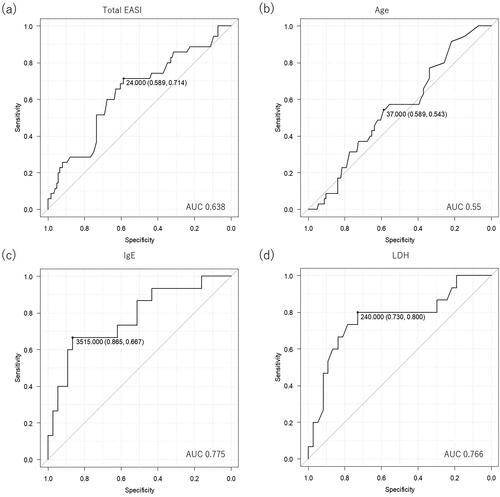
ROC analysis demonstrated that prediction of responders to upadacitinib 30 mg could be achieved by a cutoff value 3,515 IU/mL of baseline IgE with sensitivity 66.7%, specificity 86.5%, and AUC 0.775 (), indicating a fair prediction capability of baseline IgE. Furthermore, ROC analysis demonstrated that prediction of responders to upadacitinib 30 mg could be achieved by a cutoff value 240 IU/mL of baseline LDH with sensitivity 80.0%, specificity 73.0%, and AUC 0.766 (), indicating a fair prediction capability of baseline LDH.
Predictive factors for responders to upadacitinib 15 mg and 30 mg treatment in patients without previous systemic treatment
We conducted a focused analysis on patients who had not received any previous systemic treatment, including other JAK inhibitors or biologics, before starting upadacitinib treatment (Supplemental Table 1). This specific analysis was intended to assess the predictive factors for treatment response in a more uniform patient population, eliminating potential biases introduced by previous systemic treatments.
For this subgroup, a total of 128 patients in the upadacitinib 15 mg and 10 in the 30 mg who had no history of systemic treatment were analyzed.
However, upon detailed evaluation, we found no significant predictive factors for responders to upadacitinib 15 mg and 30 mg treatment in patients without previous systemic treatment. These findings suggest that the predictive factors for responders to upadacitinib may not be discernible in patients who have not been exposed to previous systemic treatments.
Discussion
In our study, we discovered several predictive factors for responders to 15 mg and 30 mg upadacitinib in Japanese AD patients at week 12. Our results contrast with those from Thyssen et al. who in their phase 3 study of 2584 patients, noted higher rates of EASI 75, IGA 0/1, and itch improvement with upadacitinib compared to placebo across various demographic and clinical variables (Citation18). The discrepancy in outcomes between our study and Thyssen et al.’s can be attributed to the unique characteristics of real-world clinical practices as opposed to controlled clinical trials. Clinical trials typically have specific criteria for participant selection, creating a more uniform patient group and standardized treatment approaches. Real-world clinical practices, however, encompass a broader patient spectrum, including varied disease severities, adherence levels, and coexisting conditions, often regulated or omitted in clinical trials. Moreover, our focus of study on Japanese patients may contribute to the observed differences.
In logistic regression analysis, the responder to 15 mg upadacitinib treatment was associated with lower basal total EASI and higher age. The association of lower basal total EASI indicates that patients with less severe clinical signs may be susceptible to the inhibitory effects of 15 mg upadacitinib. The cutoff value of basal total EASI for responders to 15 mg upadacitinib was 24 in this study, however, the AUC by this cutoff value was rather low, indicating a low accuracy. We should further find out the exact cutoff value for predicting responders in a prospective study with larger sample size.
In patients with AD, expression levels of type 2 helper T (Th2)/Th22 cytokines/chemokines (IL5, IL13, CCL13, CCL18, CCL26, IL22) in lesional skin decrease with aging, and epithelial abnormalities are normalized with aging, showing increased terminal differentiation measures (LOR, FLG) and decreased hyperplasia markers (epidermal thickness, keratin 16, and Ki67) with aging (Citation22, Citation23). It is hypothesized that older AD patients with lower Th2/Th22 responses and restored epithelial barrier might be more susceptible for the inhibitory action of upadacitinib 15 mg treatment. Related to the present results, we also previously found that higher age is associated with a higher percent reduction of PP-NRS at week 4 and 12 of upadacitinib 15 mg treatment (Citation24). However, the prediction capability of age for responders to 15 mg upadacitinib is controversial based on the very low AUC in ROC analysis. Further prospective studies should strictly inspect if higher age can predict responders to 15 mg upadacitinib. Our previous study also found that baseline IgE level was negatively correlated with percent reduction of EASI at week 4 and 12 of baricitinib 4 mg treatment, indicating the association of lower baseline IgE levels with higher treatment response to JAK1/2 inhibitor baricitinib (Citation10). Since IL-4 and IL-13 induce B cell Ig class switching to IgE, IgE levels in AD patients might reflect the activities of these type 2 cytokines. Since upadacitinib can suppress the intracellular signaling from IL-4 and IL-13 dependent on JAK1, patients with lower IgE levels, possibly with lower IL-4 and/or IL-13 activities, might be more susceptible to the inhibitory effects of upadacitinib 30 mg, leading to the higher treatment responses.
In this study, predictive factors for responders to upadacitinib were different between 15 mg versus 30 mg, lower baseline total EASI and higher age versus lower baseline IgE and LDH, respectively. The difference might be caused by different patient profiles in the two treatment groups; patients in 15 mg group had more severe clinical signs and pruritus (EASI, PP-NRS) while those in 30 mg group had higher values of laboratory parameters (IgE, TARC) indicating higher type 2-skewed immunity. The lower baseline EASI or PP-NRS in 30 mg group might be possibly caused by the higher rate of prior treatment with dupilumab, baricitinib, or 15 mg upadacitinib. The patients immediately after systemic treatments may have reduced EASI and/or PP-NRS scores, and may receive 30 mg upadacitinib treatment without sufficient wash-out time. In real-world clinical practice, upadacitinib 30 mg is likely to be applied to AD patients subsequently to systemic treatment to further improve the symptoms, which might be the reason for lower baseline clinical severity and smaller number of patients in 30 mg group of this study. Despite the higher rate of systemic pretreatments, IgE and TARC values in the 30 mg group were higher compared to 15 mg group. This is possibly because IgE and TARC values tend to decrease transiently at week 4 of upadacitinib 15 mg or baricitinib 4 mg treatment, however return to baseline levels thereafter (Citation8, Citation10).
This study has several limitations. Firstly, the number of patients treated with upadacitinib 30 mg was smaller compared to those with 15 mg. Secondly, this was a retrospective observational study, and we could not assign 15 mg or 30 mg of upadacitinib randomly to AD patients. Future interventional study should strictly identify the predictive factors for responders to each treatment dose. Thirdly, the participants of this study were limited to Japanese, and further studies should be performed on the patients with different races.
Conclusion
In conclusion, lower baseline total EASI and higher age may predict responders to 15 mg upadacitinib treatment whereas lower baseline IgE and LDH may predict responders to 30 mg upadacitinib in real-world clinical practice.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital (protocol code H-2022-945 and February 10, 2022 of approval).
Authors’ contributions
Teppei Hagino conceptualized the study, and mainly organized the manuscript. Mai Yoshida and Risa Hamada performed the statistical analyses. Naoko Kanda supervised the study. Hidehisa Saeki and Eita Fujimoto revised the manuscript.
Supplemental Material
Download PDF (144.7 KB)Disclosure statement
Hidehisa Saeki received a lecture fee and research cost from AbbVie GK. Teppei Hagino and Naoko Kanda received lecture fees from AbbVie GK.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):1–7. doi: 10.1016/j.jaad.2013.10.010.
- Nomura T, Honda T, Kabashima K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int Immunol. 2018;30(9):419–428. doi: 10.1093/intimm/dxy015.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi: 10.1111/j.1525-1470.2005.22303.x.
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4s):S65–s76. doi: 10.1016/j.jaci.2017.01.011.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi: 10.1038/s41572-018-0001-z.
- Howell MD, Kuo FI, Smith PA. Targeting the janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi: 10.3389/fimmu.2019.02342.
- Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol. 2020;21(6):783–798. doi: 10.1007/s40257-020-00548-6.
- Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167. doi: 10.1111/1346-8138.16549.
- Hagino T, Saeki H, Fujimoto E, et al. The differential effects of upadacitinib treatment on skin rashes of four anatomical sites in patients with atopic dermatitis. J Dermatolog Treat. 2023;34(1):2212095.
- Hagino T, Saeki H, Fujimoto E, et al. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023;50(7):869–879. doi: 10.1111/1346-8138.16763.
- Hagino T, Yoshida M, Hamada R, et al. Therapeutic effectiveness of upadacitinib on individual types of rash in Japanese patients with moderate-to-severe atopic dermatitis. J Dermatol. 2023;50(12):1576–1584. doi: 10.1111/1346-8138.16950.
- Hagino T, Hamada R, Yoshida M, et al. Effectiveness and safety of upadacitinib in combination with topical corticosteroids in adolescent patients with moderate-to-severe atopic dermatitis. Clin Cosmet Investig Dermatol. 2023;16:3201–3212. doi: 10.2147/CCID.S439053.
- Kamata M, Tada Y. Optimal use of jak inhibitors and biologics for atopic dermatitis on the basis of the current evidence. JID Innov. 2023;3(3):100195. doi: 10.1016/j.xjidi.2023.100195.
- Hagino T, Saeki H, Fujimoto E, et al. Background factors predicting the occurrence of herpes zoster in atopic dermatitis patients treated with upadacitinib. J Dermatol. 2023;50(10):1301–1312. doi: 10.1111/1346-8138.16879.
- Chiricozzi A, Ortoncelli M, Schena D, et al. Long-term effectiveness and safety of upadacitinib for atopic dermatitis in a real-world setting: an interim analysis through 48 weeks of observation. Am J Clin Dermatol. 2023;24(6):953–961. doi: 10.1007/s40257-023-00798-0.
- Kosaka K, Uchiyama A, Ishikawa M, et al. Real-world effectiveness and safety of upadacitinib in Japanese patients with atopic dermatitis: a two-Centre retrospective study. Eur J Dermatol. 2022;32(6):800–802. doi: 10.1684/ejd.2022.4365.
- Tran V, Ross G. A real-world Australian experience of upadacitinib for the treatment of severe atopic dermatitis. Australas J Dermatol. 2023;64(4):e352–e356.
- Thyssen JP, Thaçi D, Bieber T, et al. Upadacitinib for moderate-to-severe atopic dermatitis: stratified analysis from three randomized phase 3 trials by key baseline characteristics. J Eur Acad Dermatol Venereol. 2023;37(9):1871–1880. doi: 10.1111/jdv.19232.
- Hagino T, Yoshida M, Hamada R, et al. Early itch relief with upadacitinib predicts later skin clearance in atopic dermatitis. J Dermatolog Treat. 2024;35(1):2291317.
- Hagino T, Yoshida M, Hamada R, et al. Effectiveness of switching from baricitinib 4 mg to upadacitinib 30 mg in patients with moderate-to-severe atopic dermatitis: a real-world clinical practice in Japan. J Dermatolog Treat. 2023;34(1):2276043.
- Saeki H, Ohya Y, Furuta J, et al. Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int. 2022;71(4):448–458. doi: 10.1016/j.alit.2022.06.009.
- Nomura T, Kabashima K. Advances in atopic dermatitis in 2019-2020: endotypes from skin barrier, ethnicity, properties of antigen, cytokine profiles, microbiome, and engagement of immune cells. J Allergy Clin Immunol. 2021;148(6):1451–1462. doi: 10.1016/j.jaci.2021.10.022.
- Zhou L, Leonard A, Pavel AB, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2019;144(1):144–156. doi: 10.1016/j.jaci.2019.01.015.
- Hagino T, Saeki H, Fujimoto E, et al. The eosinophil-to-lymphocyte ratio acts as an indicator for improvement of clinical signs and itch by upadacitinib treatment in atopic dermatitis. J Clin Med. 2023;12(6):2201. doi: 10.3390/jcm12062201.

