Introduction
To the editor,
Vitiligo is an autoimmune disorder associated with high psychosocial burden due to the disease’s cosmetic disfigurement. Vitiligo affects about 0.16% of people in the United States (US) including nearly 1.52% of children, and disproportionately affects patients with skin of color (Citation1,Citation2). Treatment options are aimed at restoring skin pigmentation and include topical calcineurin inhibitors and corticosteroids and systemic therapies such as oral corticosteroids, minocycline, and cyclosporine (Citation3,Citation4). Despite the availability of treatment options, approximately 50% of patients do not receive any treatment for vitiligo (Citation5). This study aims to characterize outpatient management of vitiligo in the US utilizing data from the National Ambulatory Medical Care Survey (NAMCS).
Methods
Data from the NAMCS, an annual survey of a representative sample of US ambulatory care visits, were used to identify outpatient visits for vitiligo patients in 2012-2016, 2018, and 2019. The number of visits, age, sex, race, ethnicity, age, insurance status, specialty of the provider, and prescribed treatments (based on NAMCS drug codes) were assessed. The drug code for triamcinolone did not specify the route of administration and was assumed to be topical. Descriptive statistics were conducted using SAS version 9.04.01M7P080620. As per NAMCS policy, only one IRB is required to review the project, and the NCHS Ethics Review Board (ERB) has permitted access to the deidentified data for public use (Citation6).
Results
A total of 49 out of 190,922 outpatient visits representing an estimated 7 visits per year were conducted for vitiligo participants (0.03% of visits) with an average age of 23 years (range 1-85). Approximately 61% of participants were female, 51% were White, 39% were Hispanic/Latino, 53% had private insurance, and 39% received care from a dermatologist ().
Table 1. Characteristics of vitiligo participant visits.
The average number of medications per visit was 2.2 (range 0-14), with 22 (45%) patients not receiving any medications for vitiligo treatment. Topical corticosteroids were the most commonly prescribed agents (43%), followed by calcineurin inhibitors (20%) and systemic agents (4%) (). There were similar trends upon pediatric subgroup analysis: 53% of pediatric vitiligo visits had no medications for vitiligo treatment, 41% had topical corticosteroids, and 24% had calcineurin inhibitors ().
Figure 1. Percentage of visits that the medication was prescribed. Note: NAMCS = National Ambulatory Medical Care Survey.
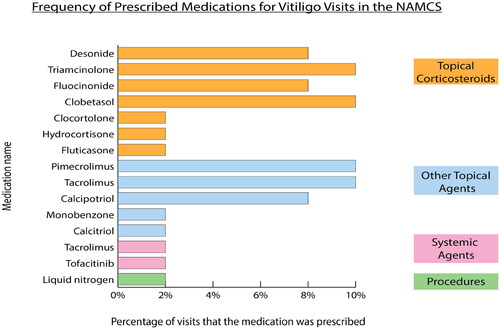
Table 2. Distribution of medications prescribed for vitiligo patient visits.
Discussion
Vitiligo outpatient visits comprise a small minority of ambulatory care visits in the NAMCS survey, and roughly equal numbers of patient visits were conducted by dermatologists and non-dermatologists. A high proportion of patients (45%) were not actively receiving any medical treatment for vitiligo. Topical corticosteroids comprise the majority of vitiligo treatment agents across both adult and pediatric groups, followed by calcineurin inhibitors, and vitamin derivatives. Systemic medications and procedural modalities comprise a small fraction of outpatient treatments for vitiligo.
Vitiligo remains an under-treated medical condition, and we find a large proportion of affected patients do not receive treatment, similar to other national averages. Similar to other studies, we find the most common agents are topical corticosteroids and topical calcineurin inhibitors; however, far fewer patients are managed on oral corticosteroids in our study compared to other national studies (0% vs 23%) (Citation5). Adverse effects associated with corticosteroids and systemic agents, including infections, osteoporosis, and diabetes may contribute to its low use (Citation7). Patterns of treatment in pediatric vitiligo have not been extensively described, and we find approximately 50% of pediatric visits included vitiligo treatment, most commonly by topical corticosteroids with no systemic agent use. A sizable proportion of vitiligo patients still do not receive treatment; barriers to vitiligo treatment include low health literacy regarding treatment options, duration of treatment, cost, and access to care. Additionally, adherence to topical medications is poor, potentially leading to frequent re-treatment and disease recurrence (Citation8).
There are limitations to this study. The information collected by NAMCS is based on a limited sample of visits rather than a number of patients, and duration of therapy was not available. Lack of resources and treatment capabilities in ambulatory care settings may limit the number of visits with procedural modalities including patients receiving phototherapy and cellular/epithelial tissue grafting. Our data were limited to certain years, and recent approvals, such as ruxolitinib, were not captured. However, the representativeness of the database strengthens the generalizability of findings. There is room for improving vitiligo treatment, and hopefully new treatments will help fill the gaps in care.
Data availability
All data sources utilized in this study are publicly available.
Disclosure statement
Feldman has received research, speaking and/or consulting support from AbbVie, Accordant, Almirall, Alvotech, Amgen, Arcutis, Arena, Argenx, Biocon, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Eurofins, Forte, Galderma, Helsinn, Janssen, Leo Pharma, Micreos, Mylan, Novartis, Ono, Ortho Dermatology, Pfizer, Regeneron, Samsung, Sanofi, Sun Pharma, UCB, Verrica, Voluntis, and vTv Therapeutics. He is founder and part owner of Causa Research and holds stock in Sensal Health. Rao is a speaker for Incyte and Amgen. All other authors have no conflicts of interest to declare.
Additional information
Funding
References
- Patel R, Pandya AG, Sikirica V, et al. Prevalence of vitiligo among children and adolescents in the United States. Dermatology. 2023;239(2):1–3. doi: 10.1159/000528180.
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159(9):986–990. doi: 10.1001/jamadermatol.2023.2162.
- Searle T, Al-Niaimi F, Ali FR. Vitiligo: an update on systemic treatments. Clin Exp Dermatol. 2021;46(2):248–258. doi: 10.1111/ced.14435.
- Gianfaldoni S, Wollina U, Tchernev G, et al. Vitiligo in children: a review of conventional treatments. Open Access Maced J Med Sci. 2018;6(1):213–217. doi: 10.3889/oamjms.2018.054.
- Rosmarin D, Soliman AM, Li C. Real-World treatment patterns in patients with vitiligo in the United States. Dermatol Ther (Heidelb). 2023;13(9):2079–2091. doi: 10.1007/s13555-023-00983-3.
- NAMCS/NHAMCS - HIPAA Privacy Rule Questions and Answers for NAMCS. Published 2019 Oct 28. https://www.cdc.gov/nchs/ahcd/namcs_hipaa_privacy.htm.
- Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33(4):289–294. doi: 10.1097/00004836-200110000-00006.
- Pathak GN, Chandy RJ, Shah R, et al. The pharmacist’s role in dermatology: patient medication adherence. J Dermatol. 2023;50(9):1099–1107. doi: 10.1111/1346-8138.16895.

