Abstract
Introduction
Dermatomyositis, systemic and cutaneous lupus erythematosus have a significantly higher prevalence in women than men, emphasizing the relevance of exploring the relationship between sex hormones and autoimmune skin diseases. This review analyzes the interplay between sex hormones and these two skin diseases.
Materials and methods
We performed an extensive literature search using the PubMed database from July to August 2023. Search terms included ‘contraceptives’, ‘pregnancy’, ‘hormone replacement’, ‘tamoxifen’, and ‘aromatase inhibitors’.
Results and Discussion
This comprehensive literature review shows that there remains considerable debate regarding the use of hormonal contraceptives and hormonal replacement therapy in individuals with autoimmune skin conditions. Nonetheless, it is well established that their use is contraindicated in patients with antiphospholipid syndrome or when antiphospholipid antibodies are positive. Individuals experiencing disease flares and uncontrolled symptoms should also avoid these interventions. Pregnancy planning should be timed to coincide with well-managed disease states to minimize obstetric and neonatal complications. Hormonal breast cancer treatment requires close skin monitoring.
Conclusion
Pregnancy, menopause, contraceptive use, hormone replacement therapy, and breast cancer treatment drugs result in substantial shifts in hormone levels. Additionally, hormone levels are altered by aromatase inhibitors and anti-estrogen medications. These fluctuations can modulate mechanisms influencing autoimmune skin abnormalities.
Introduction
Autoimmune skin diseases exhibit a higher frequency in women. Among these conditions, systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE) stand out with a significantly higher prevalence in women compared to men. SLE presents a female-to-male ratio ranging from 9-8:1. For CLE, it is lower, 3-4:1. Disease onset is often observed during periods of peak estrogen levels, suggesting a potential link between sex hormones and disease susceptibility. Similarly, classic dermatomyositis (DM) exhibits a female-to-male ratio of 2:1. In contrast, for amyopathic dermatomyositis, the ratio is higher at 5:1. Sex hormones, particularly estrogens, may play a pivotal role in modulating immune responses and affecting the risk of developing autoimmune diseases (Citation1–7).
Supporting this is that women diagnosed with SLE after menopause experience less severe organ involvement and disease flares. Furthermore, during childhood and postmenopausal periods, the prevalence of SLE in females is only double that in males, reinforcing the potential impact of hormonal variations on disease activity. These findings emphasize the relevance of exploring the relationship between sex hormones and autoimmune skin diseases (Citation3, Citation8, Citation9).
Gynecologists commonly prescribe synthetic sex hormones, such as 17α-ethinyl estradiol, for contraception or hormone replacement therapy (HRT) during menopause. Additionally, pregnancy induces significant physiological changes in sex hormone levels, with a substantial increase until delivery, followed by a sharp decline (Citation1, Citation10). Medications used for breast cancer, including anti-estrogen medications and aromatase inhibitors, also influence sex hormone levels (Citation1).
Optimizing pregnancy timing when the patient’s disease is inactive is a way to prevent flares and obstetric/fetal complications in women with rheumatic diseases. This review aims to explore the use of hormones and their impact on the disease activity of CLE and DM when managing menopausal symptoms. Planned pregnancy during disease quiescence has been associated with improved pregnancy outcomes, allowing for the discontinuation of potentially teratogenic medications before conception. The use of breast cancer medications in this patient group necessitates careful consideration because of the induction of hormonal alterations. By comprehensively examining the interplay between sex hormones and autoimmune skin diseases, this review seeks to shed light on potential therapeutic strategies and improve the clinical management of affected individuals (Citation10–12).
Material and methods
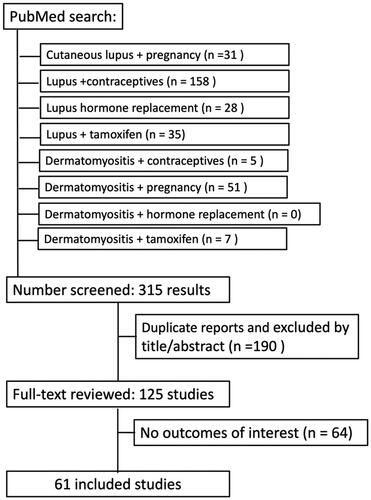
We conducted an extensive literature search using the PubMed database from July to August 2023. Search terms included ‘contraceptives’, ‘pregnancy’, ‘hormone replacement’, ‘tamoxifen’, and ‘aromatase inhibitors’. The former terms were searched together with the terms: ‘lupus’ or ‘dermatomyositis’; the one exception was ‘pregnancy’, which was searched with ‘cutaneous lupus’. Only articles from the last 10 years that were in English and pertaining to humans were included. A search of ‘contraceptives’ and ‘lupus’ returned 158 articles, whereas ‘contraceptives’ and ‘dermatomyositis’ yielded 5 articles. The search for ‘pregnancy’ and ‘cutaneous lupus’ showed 31 results, while the ‘pregnancy’ and ‘dermatomyositis’ search presented 51 results. ‘Hormone replacement’ and ‘lupus’ showed 28 results, while the search for hormone replacement with ‘dermatomyositis’ showed zero results. ‘Tamoxifen’ and ‘lupus’ yielded 18 results, ‘Raloxifen’ and ‘lupus’ yielded 7 while ‘tamoxifen’ and ‘dermatomyositis’ had 7 results. Duplicate articles have been deleted.
Initially, 125 articles were evaluated to be relevant to this literature search. Citations within these articles were checked. Articles included in this review were those that reported on the role and impact of hormones in both cutaneous autoimmune diseases. () The Center for Disease Control and Prevention guidelines chart for birth control for lupus was also included.
Results
Hormonal contraception in lupus
Effective long-acting reversible contraception and family planning methods are essential for many individuals with systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE). Given the potential risks of certain medications to the fetus, contraception is vital for women of reproductive age undergoing such treatment (Citation12–17). Notably, long-acting contraceptives like intrauterine devices (IUDs) and progestin implants are highly effective and recommended, even for nulliparous patients or adolescents without contraindications (Citation15).
Research findings are mixed regarding the impact of exogenous estrogen on developing SLE/CLE or causing disease flares, particularly in women who have either started using contraceptives or had a recent increase in dose (Citation1, Citation15, Citation18–23). Past randomized trials have demonstrated the safety of oral contraceptives containing estrogen. In women with mild or stable forms of the disease, OCPs were not linked to increased lupus flares (Citation13, Citation19, Citation20, Citation22–24). However, it is also crucial to remember the interaction between OCPs and medications used for SLE, like cyclosporine, warfarin, and mycophenolate (MMF), which reduce the efficacy of OCPs (Citation12, Citation13, Citation18).
OCPs are generally acceptable alternatives for patients with stable SLE, including those on immunosuppressive therapy and who have thrombocytopenia. Nevertheless, individuals with SLE and antiphospholipid antibodies (aPL Ab) should avoid estrogen-containing contraception due to the thrombotic risk posed by estrogen (Citation18, Citation25, Citation26).
The risk of thrombosis is low in patients with stable or inactive SLE/CLE and negative aPL Ab when prescribed hormonal contraception (Citation14, Citation27). Hence, progestin-only contraceptives, progestin implants, and depot medroxyprogesterone acetate can be safely employed in SLE patients with low disease activity (Citation23, Citation25).
Conversely, OCPs are not recommended for patients with prior thrombosis or positive aPL Ab (Citation12–15, Citation27). In comparison to OCPs, the vaginal ring provides similar or lower estrogen levels, while the patch offers higher estrogen levels, so both should also be avoided in this population (Citation18). A copper-releasing IUD is preferable in cases where patients are aPL Ab positive (Citation27). Another option is progestin-only pills, which are less effective (Citation13–15, Citation18, Citation24, Citation26) and are often discontinued due to irregular bleeding (less common with IUDs) (Citation28). Barrier methods are also viable but are less reliable (Citation15, Citation18).
Implantable and intramuscular progestins, such as depot medroxyprogesterone acetate (DMPA) injections, are safe and effective. However, long-term use of DMPA may pose a theoretical risk of osteopenia, particularly for those taking corticosteroids (Citation12–15, Citation18). Similar concerns apply to implants containing etonogestrel. Therefore, such implants should not be the primary choice for patients on long-term corticosteroids or with antiphospholipid syndrome (APS). On the other hand, DMPA might reduce the risk of hemorrhagic rupture of luteal ovarian cysts, a potentially life-threatening complication in young women on anticoagulants (Citation10).
A copper IUD is suitable for SLE patients on immunosuppressive drugs or thrombocytopenia (Citation26). The levonorgestrel IUD and progesterone implant are effective and long-lasting. The levonorgestrel IUD is associated with reduced menstrual bleeding, which benefits individuals on anticoagulant therapy (Citation13, Citation18) ().
Table 1. Summary of hormonal contraception in patients with lupus and dermatomyositis.
Hormonal contraception in dermatomyositis
Regarding contraception in dermatomyositis, there is currently a lack of research on this topic. However, it is important to emphasize effective contraceptive methods, such as hormonal contraceptives or intrauterine devices (IUDs), in patients taking teratogenic medications like methotrexate, MMF, and others (Citation29, Citation30). Long-acting reversible contraceptives, such as IUDs or subdermal progestin implants, are recommended due to their low failure rates. In cases where patients test positive for APL Ab, estrogen-combined oral contraceptive pills (OCPs) are contraindicated (Citation14).
Similar to patients with lupus, emergency contraception remains a viable option for individuals with dermatomyositis and is not contraindicated. The risks associated with emergency contraception, whether in the form of hormonal emergency contraception or copper IUDs, are substantially lower than the potential risks of an unplanned pregnancy (Citation14). Levonorgestrel is an effective and convenient emergency contraceptive that can be safely used even in patients with cardiovascular disease (Citation18) ().
Pregnancy in lupus
Unplanned pregnancies occurring in individuals with lupus raise the chance of disease exacerbation (Citation26, Citation27, Citation31). Patients with isolated cutaneous lupus erythematosus (CLE) without systemic lupus typically have normal pregnancy outcomes. Cases of CLE with anti-SSA and anti-SSB antibodies necessitate close monitoring during pregnancy for fetal heart block (Citation27, Citation31). As per guidelines from the American Heart Association, expectant mothers exhibiting anti-Ro/SSA or anti-La/SSB autoantibodies should undergo fetal echocardiography monitoring between the 16th and 18th weeks of gestation, maintaining intervals of 1 to 2 weeks until the 28th week (Citation32, Citation33). Concern for SLE should be present, especially in patients with the subacute cutaneous lupus erythematosus (SCLE) subtype, which is more commonly associated with systemic symptoms and anti-SSA (Citation13).
SLE patients should undergo renal function assessment and measurement of serological markers (serum C3/C4, anti-dsDNA titers) consistently during pregnancy to monitor for flares, thereby preventing adverse obstetrical outcomes (Citation27, Citation31). Despite advancements in treatments, SLE still poses risks during pregnancy, leading to compromised maternal and fetal outcomes (Citation13, Citation25, Citation29). Maternal risks involve disease flares, renal involvement, gestational diabetes, deep vein thrombosis, and pre-eclampsia. Fetal risks include miscarriage, intrauterine fetal demise, restricted fetal growth, preterm birth, and neonatal lupus (Citation12). Fetal exposure to anti-SSA and/or anti-SSB antibodies elevates the risk of neonatal skin lupus and heart block.
Lupus patients with only mucocutaneous symptoms show similar outcomes to healthy controls (Citation29, Citation34). Flare-ups in SLE patients are more frequent during pregnancy. There is no agreement on the specific trimester with the highest flare. They are typically moderate, involving symptoms like CLE, joint pain, and small joint arthritis (Citation13, Citation29, Citation35–37). The theoretical cause of flare-ups during pregnancy is due to elevated estrogen levels, which are linked to higher SLE risk. Risk factors for flare-ups include active disease within the 6 months before pregnancy, severe underlying comorbid conditions, active lupus nephritis, and discontinuation of hydroxychloroquine (HCQ) (Citation13).
The most significant risk factors for pregnancy loss and preeclampsia include active disease within 6 months before pregnancy, discontinuation of hydroxychloroquine, ongoing nephritis, uncontrolled hypertension, high-dose prednisone usage, or APL Ab (Citation13, Citation14, Citation34). Pregnancy should be postponed until the disease becomes inactive for at least half a year. Patients with APL Ab and a history of thrombosis should receive prophylactic anticoagulation during pregnancy and for 6 weeks after childbirth (Citation13, Citation29, Citation37). SLE-associated pregnancies usually develop smaller placentas with vascular abnormalities, ischemic injuries, and thrombotic events, heightening pregnancy loss risk (Citation13).
Lupus treatment during pregnancy
Managing lupus during pregnancy requires comprehensive care and vigilant monitoring both during pregnancy and the postpartum period (Citation12, Citation14, Citation27, Citation38). Patients should regularly use broad-spectrum sunscreen and UVB protective clothing (Citation29, Citation38). Due to low systemic absorption, topical steroids are safe for use during pregnancy (Citation13, Citation29, Citation31, Citation38–40). Mild to moderate topical steroids are preferred and should be avoided in thin areas like the face and flexural areas (Citation40). Topical calcineurin inhibitors in pregnancy are considered second-line treatment because of limited safety data (Citation25, Citation29, Citation31, Citation38–40).
HCQ is the first-line medication for CLE/SLE treatment and maintenance, while oral steroids are effective for CLE flares in pregnancy. HCQ is safe in pregnancy, although it passes through the placenta (Citation13, Citation31, Citation38, Citation40–43). HCQ use during breastfeeding is also considered safe (Citation25, Citation29, Citation40, Citation41). HCQ reduces the risk of prematurity, IUGR (intrauterine growth retardation), complete heart block, and cutaneous neonatal lupus (Citation25, Citation44).
Systemic corticosteroids are recommended if HCQ alone fails to control SLE/CLE symptoms, with non-fluorinated ones (prednisone, hydrocortisone, or prednisolone) preferred due to low placental transmission. The dosage should be the lowest possible due to the dose-dependent adverse effects such as hypertension, fluid retention, infection, gestational diabetes, preeclampsia, and avascular necrosis (Citation13, Citation14, Citation27, Citation29, Citation31, Citation38, Citation40).
Azathioprine (AZA) is used for severe CLE and SLE, with case reports supporting its safety in pregnancy for refractory cases of DLE (Citation10, Citation14, Citation25, Citation27, Citation29, Citation38, Citation40).
Sulfasalazine is an option for treatment-resistant CLE with minimal side effects (Citation39). Dapsone can be considered compatible but carries risks, especially in G6PD-deficient individuals, including dose-dependent hemolysis, methemoglobinemia, and hypersensitivity reactions. The safety of dapsone in pregnancy is reported in the literature in the treatment of malaria and leprosy (Citation29, Citation36, Citation38–40). G6PD deficiency should be assessed before prescribing dapsone (Citation40).
Continuing HCQ and starting low-dose aspirin at 12 weeks gestation for preeclampsia prevention is advised for SLE patients (Citation13, Citation14, Citation25, Citation27, Citation39–42). Cyclosporine is a secondary option for managing skin lesions. Still, it should be used cautiously due to potential placental transfer causing low birth weight (Citation38, Citation39). It is especially effective in lupus nephritis (Citation13, Citation14).
Patients who have SLE and APL Ab without thrombotic clinical events start low-dose aspirin beginning at 12 weeks until delivery to reduce the risk of preeclampsia. On the other hand, patients who meet laboratory criteria for APS and have prior consistent pregnancy complications with no history of thrombosis, as well as patients with a history of thrombotic APS, should use low-dose aspirin and unfractionated or low molecular weight heparin (LMWH) throughout pregnancy until 6-12 weeks after delivery (Citation13, Citation14, Citation27, Citation37). Warfarin is unsafe in pregnancy (Citation29).
Most medications used for lupus treatment are contraindicated in pregnancy and can also cause fertility issues. MMF is used for immunosuppression and is related to fetal malformations (Citation14, Citation16, Citation17, Citation27, Citation29, Citation31, Citation38–40) as well as methotrexate (MTX), cyclophosphamide, leflunomide, and retinoids are contraindicated during pregnancy and lactation (Citation27, Citation29, Citation31, Citation38, Citation39). MTX should be discontinued 1-3 months, and mycophenolate mofetil/mycophenolic acid at least 6 weeks before attempting pregnancy. Pregnancy should be delayed 2 years after discontinuing leflunomide (Citation10, Citation35, Citation38). Biologics like anifrolumab and belimumab have limited safety data during pregnancy (Citation13, Citation29, Citation45). Patients who have SLE and are on treatment with rituximab, anifrolumab, abatacept, and belimumab should be oriented to stop the treatment in case of conception and re-start it during lactation. These medications do not cross the placenta until the 15th week of pregnancy (Citation46) ().
Table 2. Summary of lupus and dermatomyositis treatment during pregnancy.
Pregnancy in dermatomyositis
There is limited information regarding pregnancy in individuals with dermatomyositis (DM). Approximately 14% of those with inflammatory myositis (IM) encounter this during their reproductive years (Citation29, Citation47, Citation48). Case reports and small-scale studies suggest that most DM patients with inactive disease during conception tend to stay inactive during pregnancy, leading to favorable pregnancy outcomes (Citation47). Nevertheless, the fetal prognosis is believed to worsen with the severity of the maternal disease (Citation29, Citation47, Citation49–53). Initiating treatment during pregnancy is usually unnecessary for those who have experienced extended remission. However, patients with ongoing active diseases who become pregnant are at a higher risk for obstetric complications, including IUGR, prematurity, and fetal loss. 42.9% of pregnancies are associated with active disease, resulting in fetal death (Citation54). Notably, placental abnormalities, such as substantial fibrin deposition, can emerge in cases where mothers with DM present recurrent miscarriages or preterm births (Citation55, Citation56).
It is rare for DM to start during pregnancy (Citation49, Citation56). Recent systematic reviews by Tang et al. indicated that around 16.1% of cases either worsened or manifested during the postpartum period, including 25 instances of new-onset DM (Citation30). Akalin et al. in their literature review, found that 40% of patients with a history of pregnancy developed DM during pregnancy, while 10% developed it post-partum (Citation57). Additionally, Okada et al. reported a case of juvenile DM recurrence after 20 years of remission, occurring four months post-delivery (Citation58).
The prognosis is generally poorer when the first DM symptoms appear during pregnancy or around childbirth (Citation29, Citation48, Citation50). There is contradictory data on whether pregnancy can trigger DM development (Citation56, Citation57). The immune system’s alterations during pregnancy due to hormone level fluctuations could potentially exacerbate disease activity (Citation30, Citation48, Citation50, Citation54, Citation58, Citation59). Other potential triggers include the mother’s exposure to fetal antigens and the reactivation of specific viruses due to pregnancy (Citation47, Citation54). In over half of cases, symptoms began in the first trimester, followed by the second and third trimesters (Citation51). Onset during the postpartum period was most frequent within the first week. DM onset or exacerbation can happen within 4 months post-delivery (Citation54). Nonetheless, data implies that DM may show improvement during pregnancy but can flare up after delivery (Citation29, Citation47, Citation51, Citation59–61). A study by Iago et al. demonstrated that temporary improvement was observed in half of the patients during pregnancy (Citation60).
Case reports highlight that various myositis antibodies can be activated during pregnancy (Citation47, Citation54). Patients who developed DM during pregnancy were found to have antibodies such as anti-Mi2, anti-TIF1-γ, anti-Jo-1, anti-ARS, and anti-EJ (Citation54, Citation62, Citation63). Recently, cases of MDA-5-positive DM (linked to rapidly progressive interstitial lung disease and severe skin lesions) were reported during pregnancy. Early diagnosis and treatment are correlated with better outcomes for both the mother and the baby. Active disease was significantly associated with fetal death (Citation49, Citation54–56, Citation64). Systemic involvement, including myositis and interstitial lung disease, negatively affects fetal outcomes more than skin disease alone. Delays in treatment initiation, inadequate response to steroids, elevated muscle enzymes, lung involvement, and muscle symptoms are tied to poor fetal outcomes (Citation54).
Dermatomyositis treatment during pregnancy
Due to heightened photosensitivity, sun protection is recommended (Citation29). Mid-potency topical steroids can be used for mild and localized skin diseases during pregnancy (Citation13, Citation29, Citation31, Citation38–40). Due to insufficient data, topical calcineurin inhibitors should be considered a second-line topical treatment. Similar to cutaneous lupus erythematosus, hydroxychloroquine (HCQ) is the primary systemic therapy for skin disease. For muscle or lung involvement or refractory cutaneous disease, low-dose corticosteroids should be initiated along with azathioprine to achieve a maintenance corticosteroid dose below 20 mg of prednisone daily (Citation29, Citation30, Citation48–50, Citation53, Citation54).
In severe instances, intravenous immunoglobulin (IVIG) can be used as limited research suggests its relative safety during pregnancy (Citation48, Citation54, Citation57). IVIG notably crosses the placenta significantly only after the 32nd week of gestation and is also compatible with breastfeeding (Citation29, Citation30, Citation40, Citation49, Citation54, Citation62).
Tacrolimus is a second-line treatment option and has been used during pregnancy (Citation54, Citation62). Rituximab-related literature is mixed; in the past, it was not recommended and should be discontinued at least 12 months before conception. However, in SLE studies, the drug does not cross the placenta until the 15th week of pregnancy, so treatment could be considered until pregnancy starts (Citation29, Citation39, Citation46). Immunosuppressive therapy curbs placental fibrin deposition and improves obstetric outcomes during pregnancy (Citation55). Although cyclosporine can be useful in severe cases, it can cross the placenta, entering the fetal circulation and increasing fetal exposure to other drugs, potentially contributing to low birth weight and prematurity (Citation49, Citation54). There are successful cases of concomitant cyclosporine and steroid use in polymyositis diagnosed during pregnancy (Citation65). MTX and MMF are teratogenic (Citation15, Citation17, Citation27, Citation30).
For a safe delivery, a planned C-section is advised due to the risk of rhabdomyolysis and myoglobinuria stemming from skeletal muscle damage during vaginal delivery and potential difficulties in completing vaginal delivery due to weakness (Citation62) ().
Hormone replacement therapy in lupus patients
Hormone replacement therapy (HRT) is primarily indicated to alleviate menopausal vasomotor symptoms like hot flashes and night sweats. It can also help with conditions such as atrophic vaginitis and urinary incontinence. Like OCP usage, the major concern associated with HRT is the heightened risk of arterial or venous thrombosis (Citation1). In postmenopausal women, the goal is to use the lowest effective dose for the shortest required duration to manage symptoms (Citation9). However, HRT is not consistently used in patients with SLE due to the potential to cause disease flares (Citation1, Citation14, Citation15, Citation18–20, Citation66, Citation67).
Interestingly, studies have shown a higher incidence of discoid lupus in connection with HRT. A study by Meier et al. revealed that while short-term estrogen exposure did not show a significant association, the risk of developing SLE (adjusted odds ratio [OR] 2.8; 95% confidence interval =0.9-9.0) or discoid lupus (adjusted OR 2.8; 95% CI 1.0-8.3) increased in patients exposed for two or more years (Citation68).
A meta-analysis demonstrated that women undergoing HRT have an increased risk of SLE flares, with a relative risk (RR) of 1.96 (1.51–2.56) (Citation69). The SELENA trial reported an increase in mild to moderate flares within the HRT group (Citation66). However, this study found no significant change in severe flares or mean SLEDAI scores between the HRT and placebo participants. Another study indicated that HRT did not alter SLE disease activity over two years of treatment (Citation70). Long-term use of HRT has shown that the potential risks, including stroke and breast cancer, outweigh the benefits. A considerable prospective cohort study in 1995 concluded that HRT was linked to an increased chance of postmenopausal women developing SLE (Citation1, Citation71).
Hence, HRT might trigger SLE or CLE, leading to heightened moderate flares. It is worth noting that clinical trials did not involve patients with active lupus disease (Citation68). Some studies have found no significant evidence of increased SLE incidence or flares with HRT usage (Citation1, Citation3).
Consequently, HRT could be considered for lupus patients with stable or inactive disease and a low thrombosis/APS risk. However, if aPL or APS is present, HRT should be avoided due to the impact of hormones on thrombosis and reported data showing its lack of safety (Citation1, Citation14, Citation66). The use of HRT in patients with positive aPL should be carefully considered against the probability of thrombosis and cardiovascular disease (Citation27).
Alternate options for managing climacteric symptoms aside from HRT include tibolone, which might be suitable due to its mild androgenic and progestin effects. Transdermal estradiol carries a lower risk of coagulation activation and venous thromboembolism (VTE). Using micronized progesterone, dydrogesterone, or agents that mimic pregnancy hormones also poses a neutral risk for VTE (Citation3, Citation67). Selective serotonin or noradrenaline reuptake inhibitors can be an alternative for women with contraindications to HRT, such as breast cancer or aPL. Phytoestrogens could potentially trigger flares. Lifestyle interventions remain crucial, as in women without autoimmune diseases (Citation3).
Hormone replacement therapy in dermatomyositis
There are currently no randomized clinical trials available that provide definitive evidence regarding the safety of hormone replacement therapy in individuals diagnosed with dermatomyositis. Nonetheless, in cases where no concurrent hypercoagulable state exists (such as the presence of APL Ab) and other contraindications are absent, using sex hormones to alleviate menopausal vasomotor symptoms is likely to be considered safe (Citation72).
Use of estrogen modulators in lupus patients
Selective estrogen receptor modulators (SERMs) serve as supplementary hormonal interventions for hormone-sensitive breast cancer. Generally, these medications exhibit antiestrogenic effects on specific tissues, like the breast, while paradoxically displaying estrogenic effects on other tissues, such as bone tissue (Citation5). Tamoxifen is a well-tolerated hormone treatment associated with a facial eruption and low-grade fever that exhibited poor responsiveness to self-administered fever-reducing medications (Citation73). Raloxifene is recommended for preventing and treating vertebral osteoporosis in menopausal individuals due to its antiestrogenic attributes. Although it holds potential interest in women with SLE, its use is constrained by an increased risk of VTE and potential exacerbation of climacteric symptoms (Citation3, Citation8).
Small-scale open-label investigations of SERMs have yielded inconsistent findings regarding their impact on disease severity in patients with SLE (Citation8). Both tamoxifen and raloxifene function as agonists at ER-α16, potentially contributing to an elevated occurrence of SLE in patients undergoing these therapies (Citation74). Conversely, these agents, also labeled as ‘anti-estrogens’, might ameliorate symptoms of SLE and cutaneous lupus erythematosus (CLE) (Citation75).
For instance, Mok et al. demonstrated that raloxifene significantly enhanced lumbar spinal bone mineral density and suppressed markers of bone turnover in SLE patients. Raloxifene did not escalate the risk of lupus flares or thrombosis in stable SLE patients without APL Ab (Citation8). Nevertheless, case reports suggest instances of tamoxifen-induced SCLE and acute cutaneous lupus erythematosus (ACLE), followed by complete regression of cutaneous symptoms upon discontinuing tamoxifen alone (Citation73, Citation76).
Use of estrogen modulators in dermatomyositis patients
The literature documents two cases of female dermatomyositis patients who observed an improvement in their dermatomyositis symptoms when treated with tamoxifen for breast cancer management. Upon discontinuation of the drug after four years of treatment, one of the cases experienced exacerbated rash symptoms that remained resistant to immunosuppressive therapy (Citation5).
Use of aromatase inhibitors in lupus patients
Aromatase inhibitors (AIs) are medications that reduce circulating estrogen levels as adjunctive therapy for individuals with estrogen receptor-positive breast cancer (Citation9, Citation77, Citation78). An example is anastrozole, which competitively inhibits the aromatase enzyme responsible for estrogen synthesis (Citation79). There have been instances of ocular toxicity arising from hydroxychloroquine when used concomitantly with anastrozole (Citation80). Increasing evidence indicates a connection between AIs and the onset of new autoimmune diseases such as Sjogren syndrome, anti-synthetase antibody syndrome, systemic sclerosis, discoid lupus erythematosus (DLE), and subacute cutaneous lupus erythematosus (SCLE) (Citation77). In cases where patients develop adverse skin reactions from initial AI therapy, consideration might be given to switching to an AI from a different drug class (Citation9, Citation77, Citation79, Citation81). The occurrence of CLE induced by anastrozole might appear paradoxical due to the antiestrogenic effects of AIs (Citation75).
Use of aromatase inhibitors in dermatomyositis
Emerging evidence points to a link between AIs and the initiation of new autoimmune diseases. AIs have been identified as potential triggers for anti-synthetase antibody syndrome, observed three months after initiating letrozole in a patient with a history of rheumatoid arthritis (Citation82). Among inflammatory myopathies, a case of DM was linked to anastrozole usage, with skin rash onset occurring 2.5 years post-initiation of the drug. Symptoms remitted after discontinuing the AI, with intravenous immunoglobulin, azathioprine, and hydroxychloroquine employed for dermatomyositis treatment (Citation78). However, there is a case report of a patient who experienced dermatomyositis improvement while taking anastrozole (Citation5).
Discussion
Sex hormones play a significant role in the development and functioning of both innate and adaptive immune responses. Dysregulation of these mechanisms can induce autoimmune abnormalities (Citation1). Intentional or physiologic changes in sex hormone levels could trigger autoimmune skin disease flares.
This comprehensive literature review shows that there remains considerable debate regarding the use of OCPs and HRT in individuals with autoimmune skin conditions. Nonetheless, it is well established that their use is contraindicated in patients with APS or APL Ab positive. Individuals experiencing disease flares and uncontrolled symptoms should also avoid these interventions. Pregnancy planning should be timed to coincide with inactive disease states to minimize obstetric and neonatal complications. Patients with breast cancer undergoing hormone receptor-positive treatment should monitored closely for cutaneous flares, particularly if they have a personal or family history of autoimmune diseases.
One limitation of this study is that we considered only publications from the last 10 years and focused solely on papers published in the English language. Grey literature was not included in the search, and some of the presented data relied on case reports and expert opinions due to the scarcity of controlled randomized clinical studies on this subject.
Conclusions
This study provides an extensive and updated review of the impact of sex hormones on two autoimmune skin conditions: CLE/SLE and DM. Fluctuations in sex hormone levels are characteristic of physiological states such as pregnancy and menopause. Moreover, these hormones are frequently employed as therapeutic agents in contraception and hormone replacement therapy, resulting in substantial shifts in hormone levels. Additionally, hormone levels are altered through breast cancer treatments involving aromatase inhibitors and anti-estrogen medications. These fluctuations can modulate mechanisms influencing autoimmune skin abnormalities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kim JW, Kim HA, Suh CH, et al. Sex hormones affect the pathogenesis and clinical characteristics of systemic lupus erythematosus. Front Med (Lausanne). 2022;9:1. doi: 10.3389/fmed.2022.906475.
- Nusbaum JS, Mirza I, Shum J, et al. Sex differences in systemic lupus erythematosus: epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin Proc. 2020;95(2):384–9. doi: 10.1016/j.mayocp.2019.09.012.
- Gompel A. Systemic lupus erythematosus and menopause. Climacteric. 2020;23(2):109–115. doi: 10.1080/13697137.2019.1679113.
- Williams WV. Hormonal contraception and the development of autoimmunity: a review of the literature. Linacre Q. 2017;84(3):275–295. doi: 10.1080/00243639.2017.1360065.
- Sereda D, Werth VP. Improvement in dermatomyositis rash associated with the use of antiestrogen medication [Internet]. 2006. Available from: www.archdermatol.com.
- Gerami P, Schope JM, McDonald L, et al. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54(4):597–613. doi: 10.1016/j.jaad.2005.10.041.
- El-Azhary RA, Pakzad SY. Amyopathic dermatomyositis: retrospective review of 37 cases. J Am Acad Dermatol. 2002;46(4):560–565. doi: 10.1067/mjd.2002.120620.
- Mok CC, Ying SKY, Ma KM, et al. Effect of raloxifene on disease activity and vascular biomarkers in patients with systemic lupus erythematosus: subgroup analysis of a double-blind randomized controlled trial. Lupus. 2013;22(14):1470–1478. doi: 10.1177/0961203313507987.
- Zarkavelis G, Kollas A, Kampletsas E, et al. Aromatase inhibitors induced autoimmune disorders in patients with breast cancer: a review. J Adv Res. 2016;7(5):719–726. doi: 10.1016/j.jare.2016.04.001.
- Ferguson S, Trupin L, Yazdany J, et al. Who receives contraception counseling when starting new lupus medications? The potential roles of race, ethnicity, disease activity, and quality of communication. Lupus. 2016;25(1):12–17. doi: 10.1177/0961203315596079.
- Clowse MEB, Li J, Talabi MB, et al. Frequency of contraception documentation in women with systemic lupus erythematosus and rheumatoid arthritis within the rheumatology informatics system for effectiveness registry. Arthritis Care Res (Hoboken). 2023;75(3):590–596. doi: 10.1002/acr.24803.
- Silverstein RG, Fitz V, Thornton M, et al. Contraceptive use and counseling in patients with systemic lupus erythematosus. Contraception. 2022;105:46–50. doi: 10.1016/j.contraception.2021.08.017.
- Silver R, Craigo S, Porter F, et al. Society for Maternal-Fetal Medicine Consult Series# 64: Systemic lupus erythematosus in pregnancy. Am J Obstet Gynecol. 2023;228(3):B41–B60.
- Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American college of rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care Res (Hoboken). 2020;72(4):461–488. doi: 10.1002/acr.24130.
- Sammaritano LR. Contraception in patients with rheumatic disease. Rheum Dis Clin North Am. 2017;43(2):173–188. W.B. Saunders; doi: 10.1016/j.rdc.2016.12.001.
- Al-Husayni N, Maslyanskaya S, Rubinstein TB, et al. Reproductive health care for female adolescents prescribed mycophenolate at a children’s hospital: a 10-Year retrospective cohort study. J Pediatr. 2023;253:252–258. doi: 10.1016/j.jpeds.2022.09.052.
- Abdulaziz HMM, Shemies RS, Taman M, et al. Fetal proximal and distal limb anomalies following exposure to mycophenolate mofetil during pregnancy: a case report and review of the literature. Lupus. 2021;30(9):1522–1525. doi: 10.1177/09612033211021486.
- Sammaritano LR. Contraception in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus. 2014;23(12):1242–1245. doi: 10.1177/0961203314528062.
- Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus for the OC-SELENA trial* [internet]. N Engl J Med. 2005;353(24):2550–2558. Available from: www.nejm.org. doi: 10.1056/NEJMoa051135.
- Grygiel-Górniak B, Puszczewicz MJ. The influence of endogenous and exogenous sex hormones on systemic lupus erythematosus in pre- and postmenopausal women. Prz Menopauzalny. 2014;13(4):262–266. doi: 10.5114/pm.2014.45003.
- Bernier MO, Mikaeloff Y, Hudson M, et al. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheum. 2009;61(4):476–481. doi: 10.1002/art.24398.
- Costenbader KH, Feskanich D, Stampfer MJ, et al. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56(4):1251–1262. doi: 10.1002/art.22510.
- Sanchez-Guerrero J, Karlson EW, Liang MH, et al. Past use of oral contraceptives and the risk of developing systemic lupus erythematosus. Arthritis Rheum. 1997;40(5):804–808. doi: 10.1002/art.1780400505.
- Sánchez-Guerrero J, Uribe AG, Jiménez-Santana L, et al. A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2539–2549. Available from: www.nejm.org. doi: 10.1056/NEJMoa050817.
- Birru Talabi M, Himes KP, Clowse MEB. Optimizing reproductive health management in lupus and Sjogren’s syndrome. Curr Opin Rheumatol. 2021;33(6):570–578. doi: 10.1097/BOR.0000000000000839.
- Center for Disease Control and Prevention. Summary chart of U.S. medical eligibility criteria for contraceptive use [Internet]; 2020. Available from: https://www.cdc.gov/reproductivehealth/.
- Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. 2017;76(3):476–485. doi: 10.1136/annrheumdis-2016-209770.
- Cravioto MDC, Jiménez-Santana L, Mayorga J, et al. Side effects unrelated to disease activity and acceptability of highly effective contraceptive methods in women with systemic lupus erythematosus: a randomized, clinical trial. Contraception. 2014;90(2):147–153. doi: 10.1016/j.contraception.2014.04.001.
- Wan J, Imadojemu S, Werth VP. Management of rheumatic and autoimmune blistering disease in pregnancy and postpartum. Clin Dermatol. 2016;34(3):344–352. doi: 10.1016/j.clindermatol.2016.02.006.
- Tang K, Zhou J, Lan Y, et al. Pregnancy in adult-onset dermatomyositis/polymyositis: a systematic review. American J Rep Immunol. 2022;88(5):1–7. doi: 10.1111/aji.13603.
- Lu Q, Long H, Chow S, et al. Guideline for the diagnosis, treatment and long-term management of cutaneous lupus erythematosus. J Autoimmun. 2021;123:1–16. doi: 10.1016/j.jaut.2021.102707.
- Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d.
- Clowse MEB, Eudy AM, Kiernan E, et al. The prevention, screening and treatment of congenital heart block from neonatal lupus: a survey of provider practices. Rheumatology (Oxford). 2018;57(suppl_5):V9–V17. doi: 10.1093/rheumatology/key141.
- Hamed HO, Ahmed SR, Alzolibani A, et al. Does cutaneous lupus erythematosus have more favorable pregnancy outcomes than systemic disease? A two-center study. Acta Obstet Gynecol Scand. 2013;92(8):934–942. doi: 10.1111/aogs.12158.
- Galappatthy P, Jayasinghe JDD, Paththinige SC, et al. Pregnancy outcomes and contraceptive use in patients with systemic lupus erythematosus, rheumatoid arthritis and women without a chronic illness: a comparative study. Int J Rheum Dis. 2017;20(6):746–754. doi: 10.1111/1756-185X.12996.
- Rao AG, M N, Ch S, et al. Bullous systemic lupus erythematosus in a pregnant woman with anaemia coexisting with asymptomatic hepatic haemangioma. Indian J Dermatol Venereol Leprol. 2023;89(4):585–588. doi: 10.25259/IJDVL_1299_20.
- Meiss L. Systemic lupus erythematosus in pregnancy. Consult Series; 2023.
- Vieitez Frade J, Filipe P. Lupus erythematosus: management of cutaneous manifestations during pregnancy. Dermatol Ther. 2022;35(6):e15486. doi: 10.1111/dth.15486.
- Kirchner A, Riegert M, Lake E. Current recommendations for the systemic treatment of cutaneous lupus erythematosus during pregnancy. Cutis. 2022;109(2):90-E1. doi: 10.12788/cutis.0450.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26(4):354–363. doi: 10.1111/dth.12076.
- Dima A, Jurcut C, Chasset F, et al. Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge. Ther Adv Musculoskelet Dis. 2022;14:1759720X211073001. SAGE Publications Ltd doi: 10.1177/1759720X211073001.
- Levy RA, Vilela VS, Cataldo MJ, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401–404. Available from: www.arnoldpublishers.com/journals. doi: 10.1191/096120301678646137.
- Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf. 2017;16(3):411–419. doi: 10.1080/14740338.2017.1269168.
- Barsalou J, Costedoat-Chalumeau N, Berhanu A, et al. Effect of in utero hydroxychloroquine exposure on the development of cutaneous neonatal lupus erythematosus. Ann Rheum Dis. 2018;77(12):1742–1749. doi: 10.1136/annrheumdis-2018-213718.
- Danve A, Perry L, Deodhar A. Use of belimumab throughout pregnancy to treat active systemic lupus erythematosus-a case report. Semin Arthritis Rheum. 2014;44(2):195–197. doi: 10.1016/j.semarthrit.2014.05.006.
- Dao KH, Bermas BL. Systemic lupus erythematosus management in pregnancy. Int J Womens Health. 2022;14:199–211. doi: 10.2147/IJWH.S282604.
- Silva CA, Sultan SM, Isenberg DA. Pregnancy outcome in adult-onset idiopathic inflammatory myopathy. Rheumatology (Oxford). 2003;42(10):1168–1172. doi: 10.1093/rheumatology/keg318.
- Mateus S, Malheiro M, Santos MP, et al. Dermatomyositis onset in the puerperium period. Br Med J Case Rep. 2015. Available from: http://casereports.bmj.com/.
- Chen C, Chen Y, Huang Q, et al. Case report: rapidly progressive interstitial lung disease in a pregnant patient with anti-Melanoma Differentiation-Associated gene 5 Antibody-Positive dermatomyositis. Front Immunol. 2021;12:625495. doi: 10.3389/fimmu.2021.625495.
- Gupta L, Zanwar A, Ahmed S, et al. Outcomes of pregnancy in women with inflammatory myositis: a cohort study from India. J Clin Rheumatol. 2020;26(5):165–168. doi: 10.1097/RHU.0000000000000996.
- Váncsa A, Ponyi A, Constantin T, et al. Pregnancy outcome in idiopathic inflammatory myopathy. Rheumatol Int. 2007;27(5):435–439. doi: 10.1007/s00296-006-0239-8.
- Zhong Z, Lin F, Yang J, et al. Pregnancy in polymyositis or dermatomyositis: retrospective results from a tertiary Centre in China. Rheumatology (Oxford). 2017;56(8):1272–1275. doi: 10.1093/rheumatology/kex070.
- Awatef K, Salim G, Zahra MF. A rare case of dermatomyositis revealed during pregnancy with good outcome. Pan Afr Med J. 2016;23:117. doi: 10.11604/pamj.2016.23.117.9198.
- Akiyama C, Shirai T, Sato H, et al. Association of various myositis-specific autoantibodies with dermatomyositis and polymyositis triggered by pregnancy. Rheumatol Int. 2022;42(7):1271–1280. doi: 10.1007/s00296-021-04851-1.
- Goto H, Kawahata K, Shida A, et al. Immunosuppressive therapy before and during pregnancy may improve obstetric outcomes in pregnancy complicated by dermatomyositis with anti-MDA-5 antibody positivity: a case report. Case Rep Womens Health. 2023;37:1–5. doi: 10.1016/j.crwh.2023.e00479.
- Krones C, Vu M, Popp B, et al. New onset MDA-5 positive dermatomyositis and massive perivillous fibrin deposition in third trimester of pregnancy: a case report. J Obstet Gynaecol Res. 2023;49(6):1620–1623. doi: 10.1111/jog.15625.
- Akalin T, Akkaya H, Büke B, et al. A case of New-Onset dermatomyositis in the second trimester of pregnancy: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6430156–6430156. doi: 10.1155/2016/6430156.
- Okada K, Yamanaka K, Gyobu M, et al. Well-controlled juvenile dermatomyositis over 20 years recurred after delivery. J Dermatol. 2017;44(7):855–857. doi: 10.1111/1346-8138.13548.
- Yassaee M, Kovarik CL, Werth VP. Pregnancy-associated dermatomyositis. Arch Dermatol. 2009;145(8):952–953. doi: 10.1001/archdermatol.2009.159.
- Iago PF, Albert SOC, Andreu FC, et al. “Pregnancy in adult-onset idiopathic inflammatory myopathy”: report from a cohort of myositis patients from a single center. Semin Arthritis Rheum. 2014;44(2):234–240. doi: 10.1016/j.semarthrit.2014.05.004.
- Ochiai M, Sato E, Tanaka E, et al. Successful delivery in a patient with clinically amyopathic dermatomyositis during pregnancy despite first-trimester acute exacerbation of interstitial lung disease. Mod Rheumatol. 2017;27(2):364–368. doi: 10.3109/14397595.2014.975906.
- Ito Y, Yamamoto Y, Suzuki Y, et al. Clinical and serological features and pregnancy outcomes in women with polymyositis/dermatomyositis: a case-based review. Intern Med. 2022;61(2):143–149. doi: 10.2169/internalmedicine.7924-21.
- Oya K, Inoue S, Saito A, et al. Pregnancy triggers the onset of anti-transcriptional intermediary factor 1γantibody-positive dermatomyositis: a case series. Rheumatology (Oxford). 2020;59(6):1450–1451. doi: 10.1093/rheumatology/kez527.
- Alonso-Espías M, Martínez-Sánchez N, Robles-Marhuenda A, et al. Diagnosis of amyopathic dermatomyositis after two intrauterine fetal deaths. Obstet Med. 2021;14(2):109–112. doi: 10.1177/1753495X20929507.
- Mayu S, Isojima S, Miura Y, et al. Polymyositis-Dermatomyositis and interstitial lung disease in pregnant woman successfully treated with cyclosporine and tapered steroid therapy. Case Rep Rheumatol. 2019;2019:4914631. doi: 10.1155/2019/4914631.
- Buyon JP, Petri MA, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142(12_Part_1):953–962. Available from: https://annals.org.
- Khafagy AM, Stewart KI, Christianson MS, et al. Effect of menopause hormone therapy on disease progression in systemic lupus erythematosus: a systematic review. Maturitas. 2015;81(2):276–281. doi: 10.1016/j.maturitas.2015.03.017.
- Meier CR, Sturkenboom CS, Cohen A, et al. Postmenopausal estrogen replacement therapy and the risk of developing systemic lupus erythematosus or discoid lupus. J Rheumatol. 1998;25(8):1515–1519.
- Rojas-Villarraga A, Torres-Gonzalez JV, Ruiz-Sternberg ÁM. Safety of hormonal replacement therapy and oral contraceptives in systemic lupus erythematosus: a systematic review and meta-analysis. PLoS One. 2014;9(8):e104303. doi: 10.1371/journal.pone.0104303.
- Sánchez-Guerrero J, González-Pérez M, Durand-Carbajal M, et al. Menopause hormonal therapy in women with systemic lupus erythematosus. Arthritis Rheum. 2007;56(9):3070–3079. doi: 10.1002/art.22855.
- Sanchez-Guerrero J, Liang MH, Karlson EW, et al. Postmenopausal estrogen therapy and the risk for developing systemic lupus erythematosus. Ann Intern Med. 1995;122(6):430–433. Available from: https://annals.org.
- Li R, Gebbie A, Wong R, et al. The use of sex hormones in women with rheumatological diseases. Hong Kong Med J. 2011. Available from: www.hkmj.org.
- Andrew P, Valiani S, MacIsaac J, et al. Tamoxifen-associated skin reactions in breast cancer patients: from case report to literature review. Breast Cancer Res Treat. 2014;148(1):1–5. doi: 10.1007/s10549-014-3150-0.
- Chen JY, Ballou SP. The effect of antiestrogen agents on risk of autoimmune disorders in patients with breast cancer. J Rheumatol. 2015;42(1):55–59. doi: 10.3899/jrheum.140367.
- Trancart M, Cavailhes A, Balme B, et al. Anastrozole-induced subacute cutaneous lupus erythematosus [3]. Br J Dermatol. 2008;158(3):628–629. doi: 10.1111/j.1365-2133.2007.08367.x.
- Fumal I, Danchin A, Cosserat F, et al. Subacute cutaneous lupus erythematosus associated with tamoxifen therapy: two cases [8]. Dermatology. 2005;210(3):251–252. doi: 10.1159/000083798.
- Tenti S, Giordano N, Cutolo M, et al. Primary antiphospholipid syndrome during aromatase inhibitors therapy. Medicine (Baltimore). 2019;98(13):e15052. doi: 10.1097/MD.0000000000015052.
- Bowman S, Lu H. Aromatase inhibitor-induced inflammatory myopathies: a case series. Joint Bone Spine. 2022;89(2):105308. doi: 10.1016/j.jbspin.2021.105308.
- Jung Y, Philip K, Cohen R, et al. Anastrozole-induced dermatitis: report of a woman with an anastrozole-associated dermatosis and a review of aromatase inhibitor-related cutaneous adverse events. Dermatol Ther (Heidelb). 2020;10(1):221–229. doi: 10.1007/s13555-020-00353-3.
- Hambly R, Lally A. Hydroxychloroquine toxicity and aromatase inhibitors. Br J Dermatol. 2017;177(3):882–882. doi: 10.1111/bjd.15683.
- Fisher J, Patel M, Miller M, et al. Anastrozole‐induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2008;158(3):628–629.
- Mascella F, Gianni L, Affatato A, et al. Aromatase inhibitors and anti-synthetase syndrome. Int J Immunopathol Pharmacol. 2016;29(3):494–497. doi: 10.1177/0394632016651086.